二氧化碳电还原中同位素的复杂行为
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
13c -碳同位素标记已成为一种流行的技术,用于了解二氧化碳电还原机制。然而,碳同位素表现出复杂的选择性效应,在实验设计中需要仔细考虑,以避免误解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
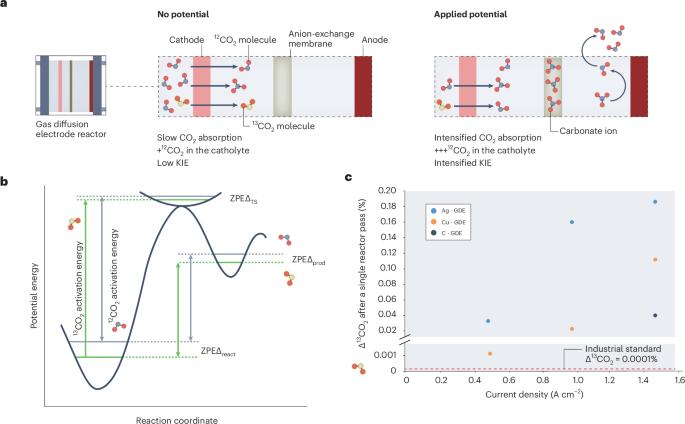
Complex behaviour of isotopes in CO2 electroreduction
Isotope labelling with 13C-carbon has become a popular technique used to understand carbon dioxide electroreduction mechanisms. However, isotopes of carbon exhibit complex selectivity effects, which need to be carefully considered in the experimental design to avoid misinterpretations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


