钠氮固溶体催化合成氨的化学环化过程
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
提出了以氢化钠为原料合成氨的工艺,并对其性能进行了研究。在压力小于1.0 MPa的条件下,NaH对氢气和氮气混合生成NH3具有催化活性。钠在各种压力和温度条件下的稳定性是催化反应的重要因素。此外,还将NaH作为化学环的反应物,在不同的过程中与N2和H2发生反应。有趣的是,在循环过程中,在小于1.0 MPa的压力下,通过化学环法可以稳定地合成NH3。作为中间相,形成了Na (NaNx)的氮固溶相。虽然可溶性氮的含量很少,约为0.02,但这一中间相对催化和化学环化过程中生成反应性原子氮具有重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
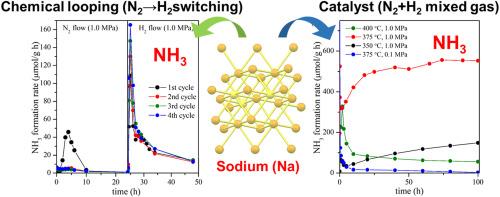
Ammonia synthesis via catalytic and chemical-looping process mediated by sodium–nitrogen solid solution
Ammonia synthesis techniques by sodium hydride are proposed, and the properties are investigated. NaH revealed a catalytic activity for the NH3 formation from mixed hydrogen and nitrogen gases below 1.0 MPa of pressure. The stability of NaH at each pressure and temperature condition was an essential factor for the catalysis. Furthermore, the NaH was used as a reactant for chemical looping process, where NaH reacted with N2 and H2 at different processes. Interestingly, NH3 can be synthesized by the chemical looping process stably under less than 1.0 MPa of pressure during the cycles. As an intermediate phase, it was indicated that a nitrogen solid solution phase of Na (NaNx) was formed. Although the amount of soluble nitrogen was small, about 0.02, this intermediate phase possessed an important role to generate reactive atomic nitrogen for the NH3 synthesis by catalytic and chemical looping processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


