s -亚硝基谷胱甘肽还原酶抑制剂治疗B细胞驱动的实验性自身免疫性脑脊髓炎的潜力
IF 2.5
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
摘要
我们之前报道了s -亚硝基谷胱甘肽(GSNO)和GSNO还原酶抑制剂,其增加内源性GSNO,通过抑制Th1和Th17效应T细胞同时促进调节性T细胞(Tregs),减轻T细胞依赖性实验性自身免疫性脑脊髓炎(EAE),多发性硬化症的动物模型。在本研究中,我们发现GSNO还原酶抑制剂(N6022)也能缓解重组人髓鞘少突胶质细胞糖蛋白(rhMOG1-125)免疫的C57BL/6小鼠B细胞依赖性EAE。发病后每日服用N6022可显著减轻EAE疾病的临床症状。N6022处理增加了脾脏中CD1dhiCD5+调节性B细胞(Bregs)的数量,抑制了B细胞效应细胞因子IL-6的表达,同时增强了调节细胞因子IL-10的表达。因此,N6022减少了脾脏中致病效应CD4+ T细胞(Th1和Th17)的数量,降低了与这些T细胞相关的促炎细胞因子(IFN-γ和IL-17a)的表达,并增加了Treg细胞的数量,同时增加了血清IL-10水平。N6022还抑制了B细胞向浆细胞的成熟,降低了血清中针对人MOG1-125 IgG的自身抗体水平。在脊髓中也进行了类似的观察,N6022治疗可调节B细胞对效应细胞因子(IL-10 >;IL-6)和调节性T细胞对效应T细胞(Treg >;Th1 / Th17)。这些发现证明GSNOR抑制剂可能在T细胞和B细胞介导的EAE和多发性硬化症中作为有效的免疫调节剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
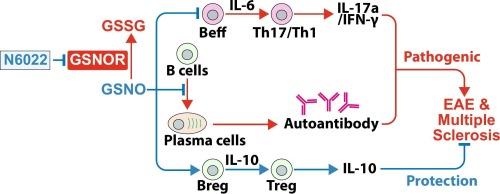
Therapeutic potential of S-nitrosoglutathione reductase inhibitor in B cell-driven experimental autoimmune encephalomyelitis
We previously reported that S-nitrosoglutathione (GSNO) and GSNO reductase inhibitors, which increase endogenous GSNO, mitigate T cell-dependent experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, by suppressing Th1 and Th17 effector T cells while promoting regulatory T cells (Tregs). In this study, we demonstrate that the GSNO reductase inhibitor (N6022) also alleviates B cell-dependent EAE in C57BL/6 mice immunized with recombinant human myelin oligodendrocyte glycoprotein (rhMOG1–125). Daily N6022 treatment following disease onset significantly reduced clinical EAE disease symptoms. N6022 treatment increased the number of CD1dhiCD5+ regulatory B cells (Bregs) in the spleen and inhibited B cell expression of the effector cytokine IL-6 while enhancing their expression of the regulatory cytokine IL-10. Accordingly, N6022 reduced the number of pathogenic effector CD4+ T cells (Th1 and Th17) in the spleen and decreased the expressions of proinflammatory cytokines associated with these T cells (IFN-γ and IL-17a), and increased the number of Treg cells with increased serum levels of IL-10. N6022 treatment also suppressed B cell maturation into plasma cells, lowering serum autoantibody levels against human MOG1–125 IgG. Similar observations were made in the spinal cord, where N6022 treatment modulated B cell expression of regulatory versus effector cytokines (IL-10 > IL-6) and the expansion of regulatory versus effector T cells (Treg > Th1/Th17). These findings document that GSNOR inhibitors may potentially serve as effective immunomodulators in both T cell- and B cell-mediated EAE and multiple sclerosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of neuroimmunology
医学-免疫学
CiteScore
6.10
自引率
3.00%
发文量
154
审稿时长
37 days
期刊介绍:
The Journal of Neuroimmunology affords a forum for the publication of works applying immunologic methodology to the furtherance of the neurological sciences. Studies on all branches of the neurosciences, particularly fundamental and applied neurobiology, neurology, neuropathology, neurochemistry, neurovirology, neuroendocrinology, neuromuscular research, neuropharmacology and psychology, which involve either immunologic methodology (e.g. immunocytochemistry) or fundamental immunology (e.g. antibody and lymphocyte assays), are considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


