嵌合抗原受体细胞疗法:将癌症治疗转化为自身免疫性疾病治疗的革命性方法
IF 8.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
目前,自身免疫性疾病主要使用广谱免疫抑制剂和单克隆抗体进行治疗,这些药物可以缓解疾病症状,但很少能治愈,而且经常伴有严重的不良反应。自身反应性B细胞在许多自身免疫性疾病的发病机制中起关键作用;然而,B细胞消耗疗法如利妥昔单抗在某些自身免疫性疾病中显示出有限的疗效,主要是由于淋巴组织和炎症部位内自身反应性B细胞的持续存在。因此,迫切需要对严重和难治性自身免疫性疾病患者进行更有效和更有针对性的治疗。在这种情况下,基因工程的最新进展促进了细胞疗法的应用,这些疗法已经从肿瘤学过渡到治疗自身免疫性疾病。利用嵌合抗原受体(CAR)工程免疫细胞的治疗已经成为一种有希望和潜在的治疗方法。针对表达cd19的B细胞在B细胞驱动的自身免疫性疾病(如系统性红斑狼疮(SLE))中的临床试验已经取得了令人鼓舞的结果,在其他治疗耐药的病例中显示出持久的缓解。此外,正在开发新的策略来扩大基于car的自身免疫治疗的治疗范围,包括嵌合自身抗体受体(CAAR)-T细胞,旨在选择性地消除自身抗原特异性B细胞,car -工程调节性T细胞(CAR-Tregs)旨在实现抗原特异性免疫调节和恢复自身耐受性。尽管取得了这些进展,但仍存在一些挑战,包括短期和长期的安全性问题、有限的体内持久性以及与个性化细胞制造相关的高成本。CAR设计方面的创新,如逻辑门控CAR、诱导式自杀开关和通用CAR结构,正在积极研究中,以提高安全性、可控性、可扩展性和临床可及性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
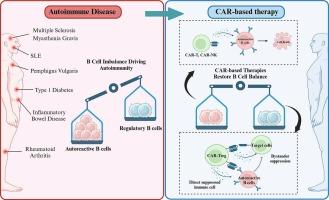
Chimeric antigen receptor cell therapy: A revolutionary approach transforming cancer treatment to autoimmune disease therapy
Currently, autoimmune disorders are predominantly managed with broad-spectrum immunosuppressive agents and monoclonal antibodies, which can alleviate disease symptoms but are rarely curative and are frequently associated with significant adverse effects. Autoreactive B cells play a key role in the pathogenesis of many autoimmune diseases; however, B-cell-depleting therapies such as rituximab have shown limited efficacy in certain autoimmune diseases, primarily due to the persistence of autoreactive B cells within lymphoid tissues and sites of inflammation. Consequently, there is an urgent need for more effective and targeted therapies for patients with severe and refractory autoimmune conditions. In this context, recent advancements in genetic engineering have facilitated the application of cell-based therapies, which have transitioned from oncology to treating autoimmune diseases. Therapies utilizing chimeric antigen receptor (CAR) engineered immune cells have emerged as a promising and potentially curative approach. Clinical trials targeting CD19-expressing B cells in B cell–driven autoimmune diseases, such as systemic lupus erythematosus (SLE), have yielded encouraging results, demonstrating durable remissions in otherwise treatment-resistant cases. In addition, novel strategies are being developed to broaden the therapeutic scope of CAR-based therapies in autoimmunity, including chimeric autoantibody receptor (CAAR)-T cells designed to eliminate autoantigen-specific B cells selectively and CAR-engineered regulatory T cells (CAR-Tregs) aimed at achieving antigen-specific immune modulation and restoration of self-tolerance. Despite these advances, several challenges persist, including short and long-term safety concerns, limited in vivo persistence, and the high costs associated with personalized cell manufacturing. Innovations in CAR design, such as logic-gated CARs, inducible suicide switches, and universal CAR constructs, are under active investigation to enhance safety, control, scalability, and clinical accessibility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


