利用异电性铜(I)配合物对烯烃和炔进行区域选择性和立体选择性β-氯酰化的一般光催化平台
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于需要使用贵重的铱光催化剂和活化烯烃作为受体,芳酰氯通过光氧化还原催化获得β-氯酰衍生物的原子转移自由基加成(ATRA)一直受到限制。在这里,我们报道了一个统一的平台,通过可见光介导的芳酰氯的ATRA,由具有广泛底物范围、可扩展性和官能团耐受性的异电性Cu(I)配合物催化,烯烃的区域选择性氯羰基化。此外,炔是可调节的底物,允许e选择性β-氯乙烯基酮的形成。该方案的合成效用通过复杂底物的功能化、产物的后修饰和药理学上相关的氟哌啶醇、塞曲司特和天然存在的哌啶生物碱(−)-sedamine的正式合成来证明。这项研究强调了异电位铜(I)配合物的排他性作用,它优于同电位铜(I)配合物,在许多ATRA过程中都是高效的,因为它具有更长的激发态寿命和适应性配体环境,可用于在title反应中由Cu(I)和Cu(II)催化的独特机制步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
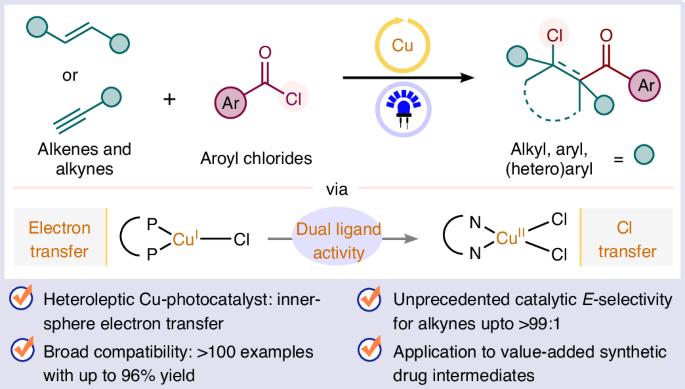
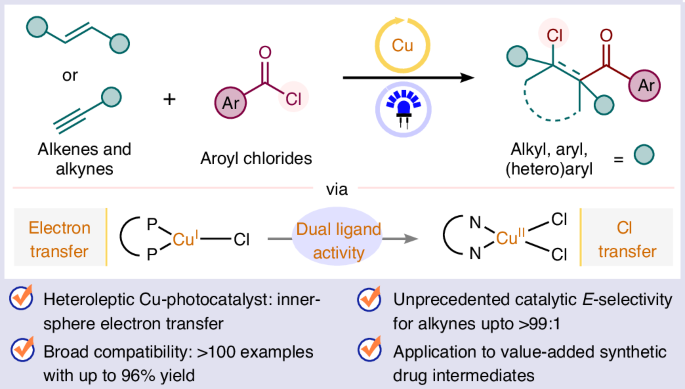
A general photocatalytic platform for the regio- and stereoselective β-chloroacylation of alkenes and alkynes using a heteroleptic copper(I) complex
Atom transfer radical addition (ATRA) of aroyl chlorides to access β-chloroacyl derivatives via photoredox catalysis remains hamstrung by the need to use precious iridium photocatalysts and activated alkenes as acceptors. Here we report a unified platform for the regioselective chlorocarbonylation of alkenes via visible-light-mediated ATRA of aroyl chlorides catalysed by a heteroleptic Cu(I) complex featuring extensive substrate scope, scalability and functional group tolerance. In addition, alkynes are amenable substrates, allowing E-selective β-chlorovinyl ketone formation. The synthetic utility of the protocol is demonstrated through the functionalization of complex substrates, post-modifications of the products and the formal synthesis of pharmacologically relevant haloperidol, seratrodast and the naturally occurring piperidine alkaloid (−)-sedamine. This study undergirds the exclusive role of a heteroleptic copper(I) complex, which outperforms homoleptic copper(I) complexes—efficient for many ATRA processes—owing to its longer excited-state lifetime and adaptive ligand environment being tailored for the distinctive mechanistic steps catalysed by Cu(I) and Cu(II) in the title reaction. There are issues hampering the wider adoption of atom transfer radical addition (ATRA) of aroyl chlorides to access β-chloroacyl derivatives via photoredox catalysis. Now, Mandal et al. report the regioselective chlorocarbonylation of alkenes and alkynes via visible-light-mediated ATRA of aroyl chlorides catalysed by a heteroleptic Cu(I) complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


