工程杂交膜囊与自体抗原和合成抗原联合作为治疗性疫苗有效抑制肿瘤复发
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
治疗性疫苗为癌症治疗提供了有希望的策略,然而,在自体新抗原中缺乏关键靶点和有限种类的合成抗原构成了重大挑战。在此,我们开发了工程杂交膜囊(MVs)作为载体平台,将来自切除肿瘤的自体膜蛋白抗原与合成抗原结合,旨在抑制肿瘤术后复发。为了增强抗肿瘤免疫,我们利用优化后的AS01佐剂,主要由QS-21和结构简化的TLR4激动剂GAP-112组成,进一步增强了自体抗原和合成抗原的免疫原性。这个基于mvs的治疗性疫苗平台,包括P(合成肽)、M(自体膜蛋白)、G (GAP112)和Q (QS-21),在包括B16-MUC1、EAC和B16-OVA肿瘤在内的三种肿瘤模型中显示出强大的抗肿瘤作用,并有效抑制肿瘤复发。其增强免疫的机制包括激活先天免疫,增强抗原摄取,诱导强大的抗体和细胞免疫反应。这些结果表明,这种基于mvs的工程混合载体平台具有作为癌症治疗通用策略的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

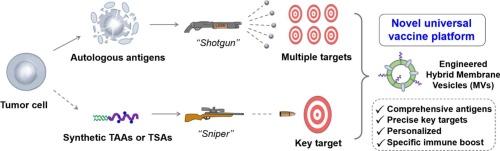
Engineered hybrid membrane vesicles combined with autologous and synthetic antigens as therapeutic vaccines to efficiently suppress tumor recurrence
Therapeutic vaccines offer promising strategies for cancer treatment, however, the lack of key targets among autologous neoantigens and limited variety of synthetic antigens pose significant challenges. Herein, we developed engineered hybrid membrane vesicles (MVs) as a carrier platform, which combines autologous membrane protein antigens derived from resected tumors with synthetic antigens, aiming to inhibit postoperative tumor recurrence. To enhance antitumor immunity, we utilized an optimized AS01 adjuvant, primarily composed of QS-21 and structurally simplified TLR4 agonist GAP-112, to further enhance the immunogenicity of the autologous and synthetic antigens. This extracellular vesicle (EV)-mimicking therapeutic vaccine platform, which includes P (synthetic peptides), M (autologous membrane proteins), G (GAP112), and Q (QS-21), demonstrates potent antitumor effects and effectively suppressed tumor recurrence in three tumor models, including B16-MUC1, EAC, and B16-OVA tumors. Its mechanisms of immune-enhancement include activating innate immunity, enhancing antigen uptake, and inducing robust antibody and cellular immune responses. These results suggest that this engineered hybrid MVs-based carrier platform holds significant potential as a universal strategy for cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


