吸入暴露于阳离子纳米塑料通过扰乱核心昼夜节律转录因子Bmal1诱导肺铁凋亡
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
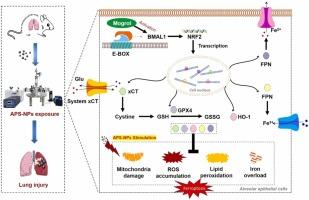
大气中纳米塑料浓度的增加引起了人们对其生物毒性的极大关注。纳米塑料的毒性往往受到其在复杂大气环境中获得的表面电荷的影响。然而,带正电的纳米塑料的肺毒性机制仍然知之甚少,缺乏有效的药理学预防和治疗策略。本研究旨在通过体内和体外模型探讨100 nm氨基改性聚苯乙烯纳米塑料(APS-NPs)的肺毒性机制。在体内,通过吸入暴露于APS-NPs的小鼠肺组织出现氧化应激和铁下垂。转录组学分析显示,与对照组相比,APS-NPs组中有566种mrna表达差异,主要与69种KEGG通路相关。这些发现表明APS-NPs通过抑制Bmal1/Nrf2/HO-1信号级联诱导肺组织铁下垂。体外,通过沉默Bmal1基因,aps - nps诱导的MLE-12细胞铁凋亡显著加重。有趣的是,预处理mogrol (Mg),一种天然的Bmal1激动剂,可以防止aps - nps诱导的肺毒性。我们的研究为纳米塑料诱导肺毒性的机制提供了新的见解,强调了Bmal1等昼夜节律转录因子在驱动铁凋亡中的作用,并提出了减轻肺损伤的潜在干预策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhalation exposure to cationic nanoplastic induces ferroptosis in the lung by perturbing core circadian transcription factors Bmal1
The increasing concentration of nanoplastics in the atmosphere has raised significant concerns regarding their biological toxicity. The toxicity of nanoplastics is often influenced by the surface charge they acquire in complex atmospheric environments. However, the mechanisms underlying lung toxicity from positively charged nanoplastics remain poorly understood, and effective pharmacological prevention and treatment strategies are lacking. This study aimed to investigate the pulmonary toxicity mechanisms of 100 nm amino-modified polystyrene nanoplastics (APS-NPs) using in vivo and in vitro models. In vivo, mice exposed to APS-NPs via inhalation exhibited oxidative stress and ferroptosis in lung tissues. Transcriptomic analysis revealed 566 differentially expressed mRNAs in the APS-NPs group compared to controls, primarily associated with 69 KEGG pathways. These findings suggest that APS-NPs induce ferroptosis in pulmonary tissues by inhibiting the Bmal1/Nrf2/HO-1 signaling cascade. In vitro, APS-NPs-induced ferroptosis in MLE-12 cells was significantly exacerbated by silencing the Bmal1 gene. Intriguingly, pre-treatment with mogrol (Mg), a natural Bmal1 agonist, protected against APS-NPs-induced lung toxicity. Our study provides new insights into the mechanisms of nanoplastic-induced lung toxicity, highlighting the role of disrupted circadian transcription factors like Bmal1 in driving ferroptosis and proposing potential intervention strategies to mitigate lung damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


