睡眠依赖性生长激素释放的神经内分泌回路
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
众所周知,睡眠可以促进组织生长和调节新陈代谢,部分是通过促进生长激素(GH)的释放,但潜在的电路机制尚不清楚。我们展示了生长激素释放在快速眼动(REM)和非快速眼动(NREM)睡眠期间增强,是如何受到表达GH释放激素(GHRH)和生长抑素(SST)的不同下丘脑神经元的睡眠-觉醒依赖活动的调节的。弓形核中的SST神经元通过抑制附近刺激GH释放的GHRH神经元来抑制GH释放,而脑室周围的SST神经元通过投射到正中隆起来抑制GH释放。生长激素释放与快速眼动睡眠期间GHRH和SST活动的强烈激增有关,但在非快速眼动睡眠期间GHRH的适度增加和SST活动的减少有关。此外,我们发现了一个负反馈通路,其中GH增强了蓝斑神经元的兴奋性并增加了觉醒。这些结果阐明了睡眠和激素调节之间双向相互作用的电路机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
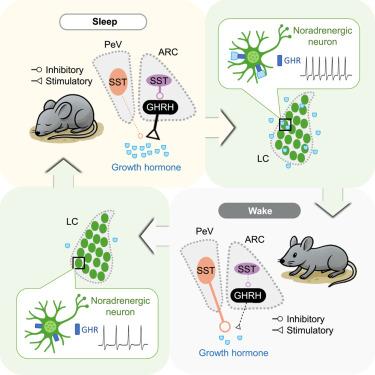
Neuroendocrine circuit for sleep-dependent growth hormone release
Sleep is known to promote tissue growth and regulate metabolism, partly by enhancing growth hormone (GH) release, but the underlying circuit mechanism is unknown. We demonstrate how GH release, which is enhanced during both rapid eye movement (REM) and non-REM (NREM) sleep, is regulated by sleep-wake-dependent activity of distinct hypothalamic neurons expressing GH-releasing hormone (GHRH) and somatostatin (SST). SST neurons in the arcuate nucleus suppress GH release by inhibiting nearby GHRH neurons that stimulate GH release, whereas periventricular SST neurons inhibit GH release by projecting to the median eminence. GH release is associated with strong surges of both GHRH and SST activity during REM sleep but moderately increased GHRH and decreased SST activity during NREM sleep. Furthermore, we identified a negative feedback pathway in which GH enhances the excitability of locus coeruleus neurons and increases wakefulness. These results elucidate a circuit mechanism underlying bidirectional interactions between sleep and hormone regulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


