在绿色荧光蛋白中插入caspase-3切割基序,设计凋亡报告基因
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
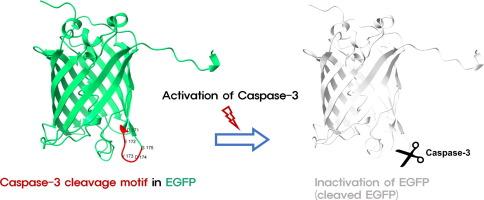
细胞凋亡是生物和动物发育的重要过程,其失调与多种疾病的进展有关。因此,检测细胞凋亡的方法对于机制研究和药物开发是必要的。为了克服传统方法检测细胞凋亡的缺点,各种基于酶介导的荧光激活的成像策略已经被开发出来。目的设计caspase-3灭活细胞凋亡荧光报告基因的新策略。方法根据结构位置和亲水性选择4个含有devd相似序列的候选EGFP突变,并通过荧光表达研究选择一个EGFP突变体。该EGFP突变体通过安全港位点在多种细胞中稳定表达。为了验证我们的凋亡报告系统,我们在凋亡诱导的表达EGFP突变的细胞中研究了蛋白水平、共定位和强度。结果staurosporine和H2O2诱导细胞凋亡,使EGFP突变体的荧光强度呈时间和浓度依赖性降低。此外,与从暗到亮的细胞凋亡报告因子(caspase-activatable GFP)相比,我们的从亮到暗系统对细胞凋亡表现出更高的敏感性。我们的系统适用于各种模型,包括其他物种。结论本方法不需要额外的多肽,易于适用于其他体系。此外,我们的细胞凋亡报告基因可用于多种研究领域以及药物筛选。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Designing an apoptosis reporter by mutagenesis-based insertion of caspase-3 cleavage motif into green fluorescence protein
Introduction
Apoptosis is an essential process for organisms and animal development, and its dysregulation is related to the progression of several diseases. Therefore, methods to detect apoptosis are necessary for mechanistic research and drug development. To overcome the disadvantages of traditional methods for detecting apoptosis, a variety of imaging strategies based on enzyme-mediated fluorescence activation have been developed.Objectives
This study aims to design a novel strategy for apoptosis fluorescent reporters which are inactivated by caspase-3.Methods
Four candidate EGFP mutations containing DEVD-similar sequences were selected by their structural positions and hydrophilicities, and an EGFP mutant was chosen by investigating fluorescence expression. This EGFP mutant was stably expressed in various cells using the safe harbor locus. To verify our apoptosis reporter system, protein levels, colocalization, and intensity were investigated in apoptosis-induced EGFP mutant-expressing cells.Results
The fluorescence intensity of the mutant EGFP was decreased in a time- and concentration-dependent manner by staurosporine and H2O2, which induce apoptosis. Furthermore, compared to a dark-to-bright reporter of apoptosis (caspase-activatable GFP), our bright-to-dark system showed greater sensitivity for apoptosis. Our system is useful in various models, including other species.Conclusion
Our method does not require additional peptides, which makes it easily adaptable to other systems. And, our apoptosis reporter may be useful in a variety of research fields as well as drug screening.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


