3-巯基丙酮酸硫转移酶位于线粒体内膜上。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
3-巯基丙酮酸硫转移酶(MPST)是一种参与产生气体递质硫化氢(H2S)的酶。与其他两种硫化氢合成酶——胱硫氨酸γ裂解酶(CSE)和胱硫氨酸β合成酶(CBS)不同,MPST存在于线粒体中。然而,MPST进入线粒体的机制及其在细胞器内的确切定位仍不清楚。利用免疫金电镜染色,我们将MPST定位在线粒体内膜上。为了研究MPST进入线粒体的途径,使用了外/内膜转位酶不同成分的药物抑制剂。我们观察到线粒体内膜上有ΜPST, TIM23的抑制阻断了MPST线粒体的进入。n端截短的ΜPST不会干扰该酶进入线粒体的能力,这表明n端前序列不会介导MPST进入线粒体。与这一发现一致,细胞质和线粒体MPST具有相似的分子量。有趣的是,n端缺失的MPST表达水平降低,表明这部分酶是蛋白质稳定所必需的。分子动力学模拟证实,酶的前39个氨基酸的缺失使蛋白质不稳定。我们的研究结果表明,MPST存在于线粒体内膜上,其进入线粒体不涉及蛋白质的n端。本文章由计算机程序翻译,如有差异,请以英文原文为准。
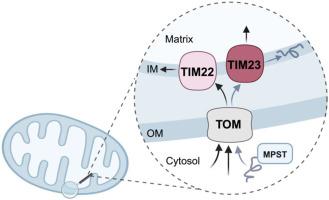
3-mercaptopyruvate sulfurtransferase resides on the inner mitochondrial membrane
3-mercaptopyruvate sulfurtransferase (MPST) is an enzyme implicated in the generation of the gasotransmitter hydrogen sulfide (H2S). Unlike, the other two H2S-synthesizing enzymes cystathionine gamma lyase (CSE) and cystathionine beta synthase (CBS), MPST is found in the mitochondria. However, the mechanisms through which MPST gains access to the mitochondria and its exact localization within this organelle remain unclear. Using immunogold electron microscopy staining, we localized MPST on the inner mitochondrial membrane. To study the pathway of mitochondrial entry for MPST, pharmacological inhibitors of different components of the translocase of outer/inner membrane were used. In line with the observation that ΜPST is found on the inner mitochondrial membrane, inhibition of TIM23 blocked MPST mitochondrial entry. Generation of N-terminally truncated forms of ΜPST did not interfere with the ability of the enzyme to gain access into the mitochondria, suggesting that an N-terminal pre-sequence does not mediate MPST mitochondrial entry. In agreement to this finding, cytosolic and mitochondrial MPST had a similar molecular weight. Interestingly, N-terminally deleted MPST exhibited reduced expression levels, indicating that this part of the enzyme is required for protein stability. Molecular dynamics simulations confirmed that deletion of the first 39 amino acids of the enzyme destabilizes the protein. Our findings reveal that MPST is present on the inner mitochondrial membrane and that its entry into mitochondria does not involve the N-terminus of the protein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


