没有放之四海而皆准的方法:对危重成人患者持续给药万古霉素血清浓度的疗效和安全性的回顾性分析显示,不同疾病严重程度的目标血清浓度不同。
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-06-20
DOI:10.1016/j.ijantimicag.2025.107556
引用次数: 0
摘要
背景:万古霉素经常被监测,但持续输注万古霉素(CIV)的目标水平是基于专家意见。很少有万古霉素浓度与CIV的疗效或安全性相关。目的:研究万古霉素稳态血清浓度与CIV治疗失败或毒性之间的关系。方法:对2010-2022年间连续接受CIV治疗的危重外科患者进行回顾性、单中心队列研究。在检测万古霉素水平、肾功能和健康状况之间的关系后,根据估计的肾小球滤过率定义了四个亚组(36)。使用主要终点(死亡率、急性肾损伤(AKI))和次要终点(临床和微生物衰竭)评估万古霉素血清浓度的失效和毒性。使用logistic和Cox回归确定结局参数的预测因子。通过双变量比较、回归模型方差分析后的事后检验和结果排序的可取性来比较浓度。浓度临界值由受体操作特征、分类和回归树分析确定。结果:共纳入922例患者。万古霉素浓度较高(前72小时平均值;特别是>25mg/L)与较高的死亡率、AKI和临床失败相关,但与微生物失败相关较少。对于SAPS bbb36,浓度为19mg/L(即20-25mg/L或19-28mg/L)。结论:对CIV期间万古霉素血清浓度进行回顾性分析,提示在选择目标浓度时应考虑ICU患者病情严重程度。靶浓度可能与SAPS呈负相关,有待在未来的前瞻性对照试验中进一步证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
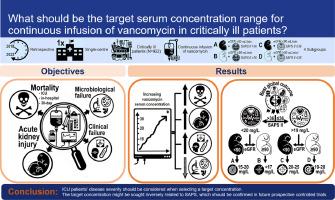
No one-size-fits-all approach: Retrospective analysis of efficacy and safety of serum concentrations of continuously administered vancomycin in critically ill adults reveals different target serum concentrations depending on disease severity
Background
Vancomycin is frequently monitored, but target levels for continuous infusion of vancomycin (CIV) are based on expert opinion. Rarely have vancomycin concentrations been correlated with therapeutic efficacy or safety of CIV.
Objectives
Associations between vancomycin steady-state serum concentrations and treatment failure or toxicity with CIV were examined.
Methods
A retrospective, single-centre cohort study was conducted of consecutive critically ill surgical patients receiving CIV between 2010 and 2022. After detecting associations between vancomycin levels, renal function and health status, four subgroups were defined based on estimated glomerular filtration rate (</≥90 mL/min/1.73m²) and Simplified Acute Physiology Score (SAPS) II (≤/>36). Failure and toxicity of vancomycin serum concentrations were assessed using primary (mortality, acute kidney injury (AKI)) and secondary (clinical and microbiological failure) endpoints. Predictors of outcome parameters were identified using logistic and Cox regression. Concentrations were compared by bivariate comparisons, post-hoc tests following analysis of variance for the regression models and desirability of outcome ranking. Concentration cut-offs were determined by receiver-operating characteristic and classification and regression tree analyses.
Results
922 patients were included. Higher vancomycin concentrations (first 72 h average, specifically >25 mg/L) were associated with higher mortality, AKI and clinical failure, but less microbiological failure. For SAPS>36, concentrations <20 mg/L (i.e. 15–20 mg/L or <17 mg/L) correlated with the best treatment outcome, for SAPS≤36 concentrations >19 mg/L (i.e. 20–25 mg/L or 19–28 mg/L).
Conclusion
Retrospective analyses of vancomycin serum concentrations during CIV suggest that ICU patients’ disease severity should be considered when selecting a target concentration. The target concentration might be sought inversely related to SAPS, which should be confirmed in future prospective controlled trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


