离子赋形剂对依那普利片“原位”形成碱性盐稳定性的影响。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-20
DOI:10.1016/j.ejpb.2025.114790
引用次数: 0
摘要
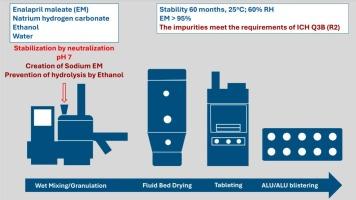
盐的形成是稳定药用物质的可能性之一。盐可以在剂型制造过程中制备,节省时间,降低成本和对环境的影响。一些研究证明了显著的依那普利马来酸盐(EM)不稳定性。EM在较高的温度和湿度下分解成二酮哌嗪(DKP)和二酸(DA)杂质。值得注意的是,有毒的DKP在pH 2 - 3时优先形成,而DA在pH大于5时主要形成。这种不稳定性引起了人们对该药治疗效果和安全性的担忧。所提出的稳定策略包括将EM“就地”转化为稳定的钠盐。这是通过将合适的离子辅料,特别是碱金属盐和乙醇基水解抑制剂掺入造粒溶液中来实现的。该方法有效地抑制了对DA的脱乙基化,提供了具有最低DKP含量的均匀片剂,确保了长期5年的稳定性。总体而言,这些片剂的降解产物含量较目前各仿制药报道的稳定性结果低,杂质含量符合ICH Q3B (R2)要求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The impact of ionic excipients on stabilization of enalapril tablets by the formation of alkaline salt “in situ”
The formation of salts is one of the possibilities of stabilizing medicinal substances. Salts can be prepared during the manufacture of the dosage form, saving time, reducing cost and environmental impact. Several studies have documented significant enalapril maleate (EM) instability. EM decomposes into diketopiperazine (DKP) and diacid (DA) impurities at elevated temperature and humidity. Notably, toxic DKP is preferentially formed at pH 2 – 3, and DA formation dominates at pH values above 5. This instability raises concerns about the therapeutic efficacy and safety of the drug. The proposed stabilization strategy involves the “in situ” conversion of EM into a stable sodium salt. This is achieved by incorporating suitable ionic excipients, specifically alkali metal salts and an ethanol-based hydrolysis inhibitor, into the granulation solution. This method effectively inhibits the deethylation to DA and provides uniform tablets with minimal DKP content to ensure long-term five-year stability. In general, these tablets show a lower content of degradation products compared to the stability results reported so far in various generics, and the amount of impurities meets the ICH Q3B (R2) requirements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


