Cu-DHM纳米酶通过级联放大免疫调节和抑制细胞凋亡来治疗皮瓣缺血再灌注损伤
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
皮瓣缺血再灌注(I/R)损伤在血流恢复后引发强烈的炎症反应和氧化应激,往往导致组织功能障碍。目前,在临床实践中还没有有效且被广泛认可的治疗策略。在皮瓣I/R损伤过程中,巨噬细胞、T细胞和中性粒细胞形成一个复杂的调控网络,共同参与炎症反应、免疫调节和组织修复。实现这三种细胞类型之间的动态平衡对于皮瓣的存活和愈合至关重要。本研究开发了一种新型Cu-DHM NP金属多酚纳米酶,该酶能有效地以级联方式放大免疫调节,抑制细胞凋亡,并治疗皮瓣I/R损伤。Cu-DHM NPs利用其优异的抗氧化性能和sod样和cat样酶活性,消除ROS,减轻细胞内氧化应激,保护线粒体功能,减少细胞凋亡。Cu-DHM NPs可以调节免疫微环境,级联并放大巨噬细胞与Naive CD4+ T细胞之间的免疫调节作用,增加M2巨噬细胞和Treg细胞的比例,减轻炎症。在动物实验中,Cu-DHM NPs下调了与炎症和细胞死亡相关的几种途径。Cu-DHM NPs抑制细胞凋亡,减少中性粒细胞浸润,减轻炎症,促进血管生成,最终提高皮瓣存活率。这种新型金属多酚纳米酶通过增强免疫调节和抑制细胞凋亡,为皮瓣I/R损伤的治疗提供了新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
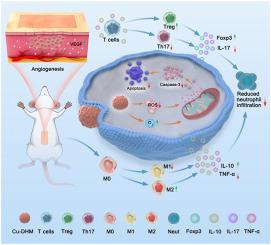
Cu-DHM nanozymes treat flap ischemia-reperfusion injury by amplifying immune modulation in a cascade manner and inhibiting cell apoptosis
Flap ischemia-reperfusion (I/R) injury triggers intense inflammatory responses and oxidative stress following blood flow restoration, often resulting in tissue dysfunction. Currently, no effective and widely recognized treatment strategies are available in clinical practice. During flap I/R injury, macrophages, T cells, and neutrophils form a complex regulatory network that jointly participates in inflammatory responses, immune modulation, and tissue repair. Achieving a dynamic balance among these three cell types is critical for flap survival and healing. In this study, a novel Cu-DHM NP metal-polyphenol nanozyme that effectively amplifies immune modulation in a cascade manner, inhibits apoptosis, and treats flap I/R injury was developed. Leveraging their excellent antioxidant properties and SOD-like and CAT-like enzyme activities, Cu-DHM NPs eliminate ROS, alleviate intracellular oxidative stress, protect mitochondrial function, and reduce apoptosis. Moreover, Cu-DHM NPs can regulate the immune microenvironment, cascade and amplify the immunomodulatory effect between macrophages and Naive CD4+ T cells, increase the proportions of M2 macrophages and Treg cells, and alleviate inflammation. In animal experiments, Cu-DHM NPs downregulated several pathways associated with inflammation and cell death. Cu-DHM NPs inhibited apoptosis, reduced neutrophil infiltration, alleviated inflammation, enhanced angiogenesis, and ultimately improved flap survival rates. This novel metal-polyphenol nanozyme offers a new strategy for treating flap I/R injury by increasing immune modulation and inhibiting apoptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


