未描述的黄链霉菌的心房素类似物,包括两个n -乙酰半胱氨酸缀合物
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Ansatrienins是一种含有三烯的c17 -苯ansamyins,由于其有效的细胞毒性而引起了人们的关注。在这项研究中,我们从黄链霉菌中鉴定出五种未被描述的心房转导素类似物:三烯霉素M - O(1-3)、N-AcCys-ansatrienin 3a(4)和N-AcCys-ansatrienin B(5)。它们的结构和相对构型通过HR-ESI-MS和NMR谱分析得以阐明,而它们的绝对构型则通过生物合成考虑和时变密度泛函理论/电子圆二色性计算得以确定。值得注意的是,trienomycin M(3)是第一个报道的含有C-25羟基的ansatrienin。化合物4和5的n -乙酰半胱氨酸(N-AcCys)片段通过硫醚连接到C-21上。抗增殖活性实验表明,三霉素N(2)对HeLa、HuCCT1和PANC-1细胞株具有显著的抗增殖活性,IC50值分别为0.81、1.76和1.84 μM。相反,化合物1、4和5是完全无活性的。这些结果表明,C-25氧化和C-21 N-AcCys修饰对细胞毒性有负面影响。这些未被描述的心房间素的发现为结构-活性关系提供了有价值的见解,并增强了我们对宿主微生物在形成天然产物中的作用的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
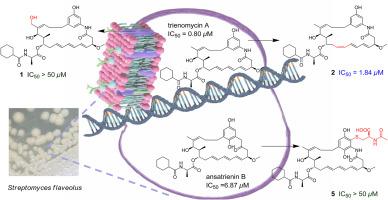
Undescribed ansatrienin analogs from Streptomyces flaveolus including two N-acetylcysteine conjugates
Ansatrienins, triene-containing C17-benzene ansamycins, have garnered attention owing to their potent cytotoxic properties. In this study, we identified five undescribed ansatrienin analogs from Streptomyces flaveolus: trienomycins M - O (1–3), N-AcCys-ansatrienin 3a (4), and N-AcCys-ansatrienin B (5). Their structures and relative configurations were elucidated using HR-ESI-MS and NMR spectroscopic analyses, whereas their absolute configurations were determined through biosynthetic considerations and time-dependent density functional theory/electronic circular dichroism calculations. Notably, trienomycin M (3) is the first reported ansatrienin with a C-25 hydroxyl group. Compounds 4 and 5 featured an N-acetylcysteine (N-AcCys) moiety attached to C-21 via a thioether linkage. Antiproliferative activity assays revealed that trienomycin N (2) exhibited significant activity against HeLa, HuCCT1, and PANC-1 cell lines, with IC50 values of 0.81, 1.76, and 1.84 μM, respectively. In contrast, compounds 1, 4, and 5 were completely inactive. These findings suggest that C-25 oxidation and C-21 N-AcCys modification negatively affected cytotoxicity. The discovery of these undescribed ansatrienins provides valuable insights into structure-activity relationships and enhances our understanding of the role of host microorganisms in shaping natural products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


