氯酮A-H是一种具有不同骨架的生物活性倍半萜类化合物。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
鉴定出8种不同骨架的氯酮A-H(1-8),包括3种壬烯型倍半萜类(1-3,属首次发现)、1种革蕊烷型倍半萜类(4)、1种松果烷型倍半萜类(5)、2种丁二烯型倍半萜类(6-7)和1种银木烷型倍半萜类(8),以及8种已知的倍半萜类(9-16)。结合光谱分析、电子圆二色性计算和x射线晶体学进行了结构表征。其中10个化合物表现出明显的抗炎活性,对lps诱导的RAW 264.7巨噬细胞一氧化氮(NO)释放有明显的抑制作用,IC50值为8.94±1.10 μM,超过阳性对照地塞米松。此外,RT-PCR和ELISA证实,10能有效降低TNF-α、IL-6、IL-1β、CXCL10、iNOS、COX-2等关键炎症介质的表达,表明其具有良好的抗炎作用。此外,通过流式细胞术分析,化合物10可以显著抑制lps诱导的RAW 264.7细胞中ROS的产生。在浓度为20 μM的h2o2诱导的斑马鱼体内,ROS的平均清除率为85.45±2.70%,与谷胱甘肽相当。本文章由计算机程序翻译,如有差异,请以英文原文为准。
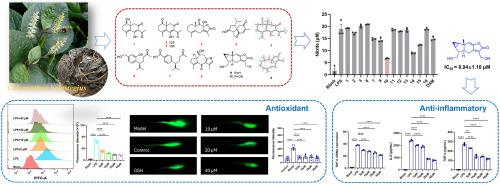
Chlorholones A−H, bioactive sesquiterpenoids with diverse skeletons from Chloranthus holostegius
Eight previously undescribed sesquiterpenoids with different skeletons, designated as chlorholones A−H (1−8), including three noreudesmane-type sesquiterpenoids (1−3, the first discovery of this type in the genus), one germacrane-type sesquiterpenoid (4), one acorane-type sesquiterpenoid (5), two cadinene-type sesquiterpenoids (6−7) and one guaiane-type sesquiterpenoid (8), together with eight known sesquiterpenoids (9−16), were identified from the whole plant of Chloranthus holostegius. A combination of spectral analysis, electron circular dichroism calculations, and X-ray crystallography were employed in the structural characterization. Among the isolated compounds, 10 exhibited notable anti-inflammatory activity, showing significant inhibition of nitric oxide (NO) release in LPS-induced RAW 264.7 macrophages, with an IC50 value of 8.94 ± 1.10 μM, surpassing the positive control, dexamethasone. Furthermore, 10 effectively reduced the expression of key inflammatory mediators, including TNF-α, IL-6, IL-1β, CXCL10, iNOS, and COX-2, as confirmed by RT-PCR and ELISA, indicating its promising anti-inflammatory effects. Additionally, compound 10 demonstrated significant inhibition of LPS-induced ROS production in RAW 264.7 cells, as evidenced by flow cytometry analysis. It also achieved an average ROS clearance rate of 85.45 ± 2.70 % in H2O2-induced zebrafish at a concentration of 20 μM, comparable to glutathione.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


