Al3BC3氧化法制备多孔Al2O3
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
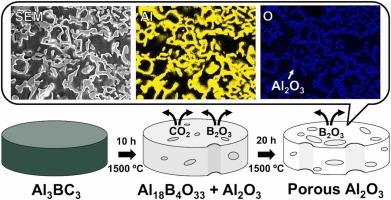
本文介绍了一种以Al3BC3粉末为原料,采用直接成孔法氧化Al3BC3制备多孔Al2O3的新方法。Al3BC3在1150℃开始氧化生成Al18B4O33,由于B2O3和CO2的挥发(升华)形成多孔微观结构。在长时间高温加热下,Al18B4O33分解,B2O3进一步挥发,生成孔隙率高的单相Al2O3。孔隙率最高为71.6%,本研究中存在1 ~ 4µm的小孔隙和20µm的大孔隙。通过改变热处理温度可以控制其孔径大小。Al2O3的抗弯强度为34.7 ~ 36.3 MPa,优于其他多孔陶瓷,但其孔隙率较高。这种高抗弯强度可能是由于Al2O3的高均匀性和多孔Al2O3网络结构的形成,其中颗粒在高温下牢固地结合在一起。所提出的方法很容易提供均匀的多孔Al2O3样品,而不需要将成孔剂混合或分散到基体材料中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Preparation of Porous Al2O3 via Oxidation of Al3BC3
This paper describes a new, simple, and convenient method to prepare porous Al2O3 through oxidation of Al3BC3 via a direct pore-forming approach using only Al3BC3 powder as a starting material. Oxidation of Al3BC3 to Al18B4O33 started at 1150 °C, and a porous microstructure was formed because of the volatilization (sublimation) of B2O3 and CO2. When heated at high temperature for a long period, Al18B4O33 decomposed, accompanied by further volatilization of B2O3, resulting in monophasic Al2O3 with high porosity. The highest porosity was 71.6%, and small pores of 1–4 µm and large pores of 20 µm were observed in the present study. The pore size could be controlled by varying the heat-treatment temperature. The bending strength was 34.7–36.3 MPa, which is superior to those of other porous ceramics despite the high porosity of the Al2O3. This high bending strength is likely due to the high homogeneity of the Al2O3 and the formation of a porous Al2O3 network structure in which the particles are firmly bonded together at high temperatures. The proposed method easily provides a homogeneous porous Al2O3 sample without awkward processes such as mixing or dispersing a pore-forming agent into the matrix material.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


