青少年慢性社会失败和社会陪伴对情绪和社会行为的性别依赖效应及脑区OT/Fos和AVP/Fos双标记细胞的数量
IF 2.5
3区 医学
Q2 BEHAVIORAL SCIENCES
引用次数: 0
摘要
本研究探讨了慢性社会失败应激(CSDS)对青春期雌雄田鼠抑郁样行为的影响。此外,我们还研究了CSDS期间社会陪伴在缓解CSDS诱导的抑郁样行为变化以及催产素(OT)和17个精氨酸抗利尿素(AVP)神经元活动中的作用。CSDS暴露降低了两性对蔗糖的偏好和社会互动。然而,CSDS仅在雌性中增加了尾悬试验(TST)和强迫游泳试验(FST)的静止时间。社会性:两性对蔗糖的偏好和社会互动增加,而雌性的TST和FST的静止时间减少。这些结果表明,社会陪伴可能会增强青春期田鼠的应激恢复能力,尤其是雌性田鼠。在神经水平上,CSDS导致女性室旁核(PVN)、视上核(SON)、终纹床核(BNST)和内侧杏仁核(MeA)中OT/c- fos阳性细胞减少,而男性这些区域的激活增加。社会陪伴在女性中逆转了这些变化,但在男性中没有,这表明社会互动对女性OT神经元活动的伴随作用更强。相反,CSDS降低了男性PVN、SON、BNST和MeA中AVP/c- fos阳性细胞,表明AVP在男性应激反应中有更大的参与。社会陪伴在男性中逆转了这些与avp相关的变化,但在女性中没有。这些发现强调了性别依赖于CSDS的神经和行为反应,并确定了社会互动促进青春期压力恢复的潜在机制。PVN、SON、BNST和MeA中的OT和AVP活性可能是社会陪伴保护作用的分子基础,影响行为和神经结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
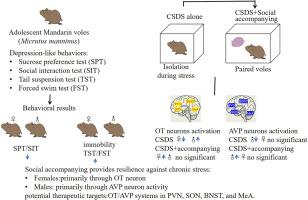
Sex-dependent effects of adolescent chronic social defeat and social accompanying on emotional and social behaviors and the numbers of OT/Fos and AVP/Fos dual-labelled cells in brain regions of the mandarin vole (Microtus mandarinus)
This study investigated the effects of chronic social defeat stress (CSDS) on depression-like behaviors in adolescent male and female Mandarin voles (Microtus mandarinus). Additionally, we examined the role of social accompanying during CSDS in mitigating CSDS-induced changes in depression-like behaviors, as well as activities of oxytocin (OT) and 17 arginine vasopressin (AVP) neurons. CSDS exposure reduced sucrose preference and social interaction in both sexes. However, CSDS increased immobility time in the tail suspension test (TST) and forced swim test (FST) only in females. Social accompanying increased sucrose preference and social interaction in both sexes, while reducing immobility time in the TST and FST exclusively in females. These results suggest that social accompanying may enhance stress resilience in adolescent voles of both sexes, particularly in females. At the neural level, CSDS led to a decrease in OT/c-Fos-positive cells in the paraventricular nucleus (PVN), supraoptic nucleus (SON), bed nucleus of the stria terminalis (BNST), and medial amygdala (MeA) in females, but increased activation in these regions in males. Social accompanying reversed these changes in females, but not in males, indicating a stronger accompanying effect of social interaction on OT neuron activity in females. Conversely, CSDS decreased AVP/c-Fos-positive cells in the PVN, SON, BNST, and MeA in males, suggesting greater AVP involvement in the male stress response. Social accompanying reversed these AVP-related changes in males, but not in females. These findings highlight sex-dependent neural and behavioral responses to CSDS and identify potential mechanisms through which social interaction promotes stress resilience during adolescence. OT and AVP activity in the PVN, SON, BNST, and MeA may serve as molecular substrates for the protective effects of social accompanying, shaping both behavioral and neural outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physiology & Behavior
医学-行为科学
CiteScore
5.70
自引率
3.40%
发文量
274
审稿时长
47 days
期刊介绍:
Physiology & Behavior is aimed at the causal physiological mechanisms of behavior and its modulation by environmental factors. The journal invites original reports in the broad area of behavioral and cognitive neuroscience, in which at least one variable is physiological and the primary emphasis and theoretical context are behavioral. The range of subjects includes behavioral neuroendocrinology, psychoneuroimmunology, learning and memory, ingestion, social behavior, and studies related to the mechanisms of psychopathology. Contemporary reviews and theoretical articles are welcomed and the Editors invite such proposals from interested authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


