穿心莲内酯通过Th17细胞分化介导的糖酵解途径对ova刺激小鼠的抗哮喘作用。
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:哮喘一直被认为是一个主要的全球健康问题。尽管现有的治疗方法疗效显著,但病情恶化的发生率和死亡率仍然高得惊人。穿心莲内酯(Andrographolide, AG)是从中药穿心莲中提取的,具有不同的平喘作用机制。但目前还没有关于AG通过糖酵解在哮喘中的调节作用的研究。本文旨在探讨糖酵解途径在AG抑制哮喘中的潜在作用及机制。方法:将动物随机分为6组:对照组、OVA模型组、AG (0.1mg/kg)组、AG (0.5mg/kg)组、AG (1mg/kg)组和DEX (2mg/kg)组。建立OVA模型,分别采集小鼠BALF、血清和肺组织进行ELISA、rt-PCR、western blot和免疫荧光染色。利用网络药理学和流式细胞术分析和验证AG糖酵解治疗哮喘的潜在靶点。结果:AG能减弱ova诱导的肺组织中HK2、乳酸和PKM2的产生以及血清和肺组织中IL-1β的产生;AG抑制ova刺激下肺组织Glut-1、LDHA、PKM2 mRNA的表达;AG抑制ova介导的肺组织HK2、Glut-1、LDHA、PKM2磷酸化、IL-17的表达;AG还可减轻肺组织中PKM2的表达。网络药理学揭示了IL-1β等36个靶基因及AG抑制Th17细胞分化的潜在机制。结论:我们认为AG通过阻断糖酵解通路的激活,特别是通过靶向Th17细胞分化,抑制ova刺激小鼠哮喘的炎症反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
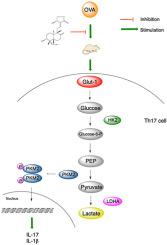
Anti-asthmatic effects of andrographolide on OVA-stimulated mice via Th17 cell differentiation-mediated glycolytic pathway
Background
Asthma has always been considered a major global health issue. Despite the significant efficacy of the established treatment, the incidence of exacerbation and mortality remains alarmingly high. Andrographolide (AG), extracted from the traditional Chinese herb Andrographis paniculate, has been proved to be anti-asthmatic with different mechanisms. But there have been no studies involving the regulatory function of AG in asthma through glycolysis. Herein, we aim to explore the potential effects and mechanism of glycolytic pathway in AG inhibition of asthma.
Methods
Animals were randomly divided into 6 groups: a control group, an OVA model group, AG (0.1 mg/kg) group, AG (0.5 mg/kg) group, AG (1 mg/kg) group and DEX (2 mg/kg) group. The OVA models were established and the BALF, serum and lung tissue of the mice were collected separately for the administration of ELISA, rt-PCR, Western blot and immunofluorescence staining. Network pharmacology and flow cytometry were also utilized to analyze and verify the potential targets of AG in treatment of asthma by glycolysis.
Results
AG attenuated the OVA-induced production of HK2, lactate and PKM2 in lung tissue and the production of IL-1β in serum and lung tissue; AG restrained the OVA-stimulated expression of mRNA of Glut-1, LDHA and PKM2 in lung tissue; AG inhibited the OVA-mediated protein expression of HK2, Glut-1, LDHA, phosphorylation of PKM2, IL-17 in lung tissue; AG also alleviated the expression of PKM2 in lung tissue. Network pharmacology revealed 36 target genes including IL-1β and potential mechanism Th17 cell differentiation which was suppressed by AG.
Conclusions
We conclude that AG inhibits the inflammatory response of asthma in OVA-stimulated mice by blocking the activation of glycolytic pathway, especially by targeting Th17 cell differentiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


