白细胞介素-18 (IL-18)通过延长半衰期和抵抗IL-18结合蛋白(IL-18BP)来增强抗癌治疗潜力
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
重组白细胞介素-18 (IL-18)作为适应性和先天免疫细胞的强大激活剂和IFN-γ的有效生产者,有望成为肿瘤治疗的一种药物。然而,由于天然IL-18抑制剂IL-18结合蛋白(IL-18BP)的快速上调,以及不与IL-18BP结合的活性IL-18的快速全身清除,临床成功受到限制。为了克服这些限制,我们设计了人类IL-18和小鼠同源物对IL-18BP的抗性,并通过与Fc支架融合延长半衰期,目的是创造一种改进的基于IL-18的治疗方法。具有一系列效力的IL-18变体没有检测到与IL-18BP的结合,并且在存在超生理水平的IL-18BP时保持充分的生物活性。将小鼠同源物与“裸”IL-18BP抗性工具IL-18变体进行比较的药代动力学研究证实了半衰期延长的重要性。重要的是,与裸IL-18变体相比,小鼠Fc同源物表现出更强、更持久的TH1细胞因子产生和细胞毒性NK细胞和效应CD8+ T细胞的扩增。值得注意的是,在多同基因肿瘤模型中,与用载体处理的小鼠相比,每周给药一次的小鼠工程IL-18变体显示出强烈的肿瘤生长抑制作用,并提高了生存率。在人源化小鼠中给药的人IL-18变体反映了小鼠同源物在体内的发现,显示出延长的药代动力学和持久的血清TH1细胞因子上调。这些有希望的临床前结果突出了工程IL-18溶液抵抗IL-18BP结合并延长半衰期,释放其持续抗癌免疫反应的全部潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
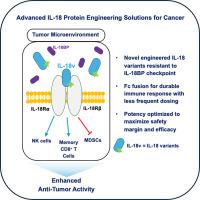
Interleukin-18 (IL-18) engineered for half-life extension and resistance to IL-18 binding protein (IL-18BP) to enhance anti-cancer therapeutic potential
Recombinant interleukin-18 (IL-18) holds promise as a therapeutic for oncology, as a robust activator of adaptive and innate immune cells and a potent producer of IFN-γ. However, clinical success has been limited due to rapid upregulation of the natural IL-18 inhibitor, IL-18 binding protein (IL-18BP), and fast systemic clearance of active IL-18, unbound to IL-18BP. To overcome these limitations, human IL-18 and mouse orthologs were engineered with resistance to IL-18BP and extended half-life by fusion to an Fc scaffold, with the goal of creating an improved IL-18-based therapeutic. IL-18 variants with a range of potencies showed no detectable binding to IL-18BP and retained full biological activity in the presence of super-physiological levels of IL-18BP. Pharmacokinetic studies comparing the mouse orthologs with a “naked” IL-18BP resistant tool IL-18 variant confirmed the importance of half-life extension. Importantly, the mouse Fc orthologs exhibited stronger and more durable TH1 cytokine production and expansion of cytotoxic NK cells and effector CD8+ T cells when compared to the naked IL-18 variant. Remarkably, mouse engineered IL-18 variants administered once a week demonstrated strong tumor growth inhibition and improved survival compared to vehicle treated mice in multiple syngeneic tumor models. Human IL-18 variants dosed in humanized mice mirrored in vivo findings from mouse orthologs, displaying extended pharmacokinetics and durable upregulation of serum TH1 cytokines. These promising preclinical results highlight engineered IL-18 solutions that resist IL-18BP binding and extend half-life, unlocking its full potential for sustained anti-cancer immune responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


