K2S2O8/ dmso介导的木香酸与亚硫酸盐的甲基化/磺化一锅法合成磺酰基甲基邻苯酞。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以K2S2O8/ dmso为催化剂,通过双甲基化和磺化两种途径合成了多种磺酰基甲基三氧邻苯酞。在这个有效的反应中,二甲基亚砜同时作为溶剂和亚甲基源,在开容器条件下形成一个碳-硫单键、一个碳-氧单键和两个碳-碳单键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
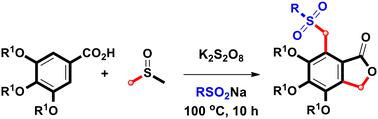
One-pot synthesis of sulfonylmethyl phthalides via K2S2O8/DMSO-mediated methylenylation/sulfonylation of eudesmic acid with sulfinates†
A K2S2O8/DMSO-mediated one-pot reaction of eudesmic acid with sodium sulfinates was developed for the synthesis of various sulfonylmethyl trioxygenated phthalides via double methylenation and sulfonylation pathways. In this effective reaction, dimethyl sulfoxide was used as both a solvent and a methylene source through the formation of one carbon–sulfur single bond, one carbon–oxygen single bond, and two carbon–carbon single bonds under open-vessel conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


