黑姜植物化合物的抗癌活性研究。(前贝克):用计算机方法
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
背景:黑姜(BG)、山柰(kaempiia parviflora)的主要成分。(Baker))显示出多种生物效应,特别是潜在的抗癌活性。此外,计算机计算方法为从药用植物中发现新的候选治疗药物提供了强有力的策略,为解决日益增加的全球癌症负担提供了创新的解决方案。方法采用液相色谱-质谱法对BG提取物中的植物成分进行初步鉴定。利用AutoDock Vina软件对35个BG化合物进行分子对接,筛选出11个抗癌靶点。结果5种BG化合物KP1、KP2、viscumneside VI和5-羟基-7-甲氧基黄酮(5-Hydroxy-7-methoxyflavone, 5H7M)与多种抗癌靶点的相互作用较参比药物最强。KP1对HDAC6(−8.6 Kcal/mol)、EGFR(−9.5 Kcal/mol)、mTOR(−9.7 Kcal/mol)、PI3K(−10.3 Kcal/mol)和PD1(−7.9 Kcal/mol)具有良好的结合亲和力(BA)。同时,viscumneside VI对HDAC6、CDK2、EGFR、PI3K和PD1 5个靶标(−7.9 ~−9.4 Kcal/mol)表现出良好的BA, 5H7M对DHFR、PI3K、KDR和PDL1 4个靶标(−9.5 ~ 10.0 Kcal/mol)表现出良好的结合亲和力。KP2对KDR (- 9.8 Kcal/mol)和与KP1相似的5个靶标(- 8.3 ~ - 9.9 Kcal/mol)表现出良好的结合亲和力和氢键(HB)形成。植物化合物KP1、KP2、viscumneside VI和5H7M与DHFR、HDAC6、CDK2、EGFR、PI3K、ALK和KDR的氨基酸残基表现出类似于参比药物的相互作用(HB、静电和疏水)。此外,与抗癌药物相比,这些植物化合物显示出良好的硅ADMET谱。结论这些潜在的植物化合物需要进一步分离、合成和深入研究,以开发新的抗癌药物,特别是KP1和KP2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
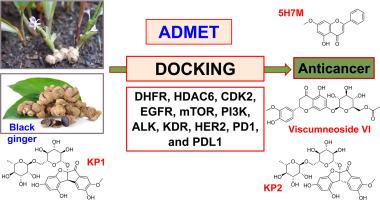
Anticancer activity of phytocompounds of black ginger (Kaempferia parviflora Wall. Ex Baker): In silico approach
Background
The main components of black ginger (BG, Kaempferia parviflora Wall. Ex Baker) show diverse biological effects, especially potential anticancer activity. Furthermore, in silico computational approaches offer a powerful strategy for discovering novel therapeutic candidates from medicinal plants, providing an innovative solution to address the increasing global burden of cancer.
Methods
Tentative identification of phytocompounds of BG extracts was performed using the LC-MS method. Thirty-five phytocompounds of BG were screened using molecular docking with AutoDock Vina software against eleven anticancer targets.
Results
Five BG phytocompounds KP1, KP2, Viscumneoside VI, and 5-Hydroxy-7-methoxyflavone (5H7M) showed the strongest interactions with multiple anticancer targets compared to the reference drugs. KP1 showed good binding affinity (BA) against five targets (HDAC6 (−8.6 Kcal/mol), EGFR (−9.5 Kcal/mol), mTOR (−9.7 Kcal/mol), PI3K (−10.3 Kcal/mol), and PD1 (−7.9 Kcal/mol)). Meanwhile, Viscumneoside VI exhibited good BA against five targets (HDAC6, CDK2, EGFR, PI3K, and PD1 (−7.9 to −9.4 Kcal/mol)), and 5H7M showed good binding affinity against four targets (DHFR, PI3K, KDR, and PDL1 (−9.5 to 10.0 Kcal/mol)). In particular, KP2 showed good binding affinity and hydrogen bond (HB) formation against six targets, including KDR (−9.8 Kcal/mol) and five targets similar to KP1 (−8.3 to −9.9 Kcal/mol). Phytocompounds KP1, KP2, Viscumneoside VI, and 5H7M exhibited some interactions (HB, electrostatic, and hydrophobic) with amino acid residues of DHFR, HDAC6, CDK2, EGFR, PI3K, ALK, and KDR similar to the reference drugs. Furthermore, these phytocompounds showed good in silico ADMET profiles compared to anticancer drugs.
Conclusion
These potential phytocompounds need to be isolated, synthesized and researched more in-depth for the development of new cancer drugs, especially KP1 and KP2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


