Mn-Co氧化物与活性炭载体协同作用促进甲苯在MnCo/AC上的催化氧化
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
活性焦炭具有良好的多孔结构,作为吸附剂和催化剂载体在大气污染物治理中得到了广泛的应用。在本工作中,合成了负载在AC上的Mn-Co氧化物来去除甲苯。优化后的Mn1Co9/AC在238℃下的甲苯去除率达到90%以上,与一些纯金属氧化物催化剂相当,但成本较低。此外,在240℃条件下,即使SO2浓度为500 ppm或H2O浓度为10 vol%, Mn1Co9/AC对甲苯的去除率也保持在80%以上,具有工业应用潜力。表征结果表明,Mn1Co9/AC具有强的金属氧化物-载体相互作用,增强了对反应物的吸附,活性成分高度分散,加速了电子传递。此外,MnOx和CoOx之间的协同作用促进了活性氧的形成,包括丰富的表面吸附氧、高迁移率的氧空位和晶格氧、价态较高的金属离子,从而具有优异的还原性。结合红外光谱分析,甲苯→苯甲醇→苯甲醛→苯甲酸在较低温度下进行初始氧化,苯甲酸→马来酸酐→碳酸盐→CO2 + H2O在较高温度下进行进一步氧化。O2通过不断补充消耗的活性氧,促进了甲苯的吸附和随后的中间体氧化,特别是苯甲醇的氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
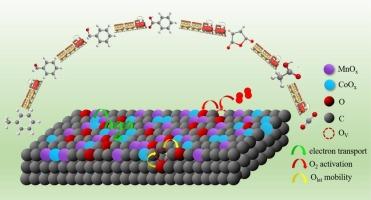
Boosting catalytic oxidation of toluene on MnCo/AC by synergistic effect of Mn-Co oxides and AC support
Activated coke (AC), with well-developed porous structure, has been extensively applied as adsorbents and support of catalysts for air pollutants control. In this work, Mn-Co oxides supported on AC were synthesized for toluene removal. The optimal Mn1Co9/AC exhibited remarkable toluene removal efficiency above 90 % at 238 ℃, comparable to some pure metal-oxides catalysts but with a lower cost. Besides, Mn1Co9/AC sustained toluene removal efficiency above 80 % even with 500 ppm SO2 or 10 vol% H2O at 240 ℃, implying its potential of industrial application. Characterizations revealed that the strong metal oxides-support interaction endowed Mn1Co9/AC with enhanced adsorption of reactants, highly-dispersed active components and accelerated electron transport. Additionally, the synergy between MnOx and CoOx facilitated the formation of active oxygen species, including abundant surface adsorbed oxygen, oxygen vacancies and lattice oxygen with high mobility, metal ions with higher valence, and thus superior reducibility. Combined with FTIR, the initial oxidation of toluene → benzyl alcohol → benzaldehyde → benzoic acid proceeded at lower temperature, while the further oxidation of benzoic acid → maleic anhydride → carbonates → CO2 + H2O occurred with elevated temperature. And O2 facilitated both toluene adsorption and subsequent oxidation of intermediates, especially for benzyl alcohol oxidation, via continuous replenishment of consumed active oxygen species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


