低温碱熔-酸步浸长焰煤生产超低灰分煤的除杂行为及机理
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
煤,除了金刚石和石墨,是自然界中最大的碳物质来源。长焰煤具有较高的化学反应活性,将成为煤基功能碳材料的重要前驱体。然而,煤的深度脱硫和超纯制备已成为影响其发展的主要瓶颈。以新疆长焰煤为原料,研究了低温碱熔酸步浸法脱除长焰煤中的杂质生产超低灰分煤的机理。进一步探讨了杂质去除的行为和机理。结果表明,碱熔提取灰分可达0.80%,收率可达73.26%。经HNO3浸出富集后,可获得灰分0.009%的超低灰分煤。最大脱矿率为99.42%。分析了Si/S/Fe/Al元素的迁移机理。部分Si/S元素作为氧化物被去除,少量Si/S与NaOH反应生成Na2SiO3和Na2S等氧化物。Fe/Al元素生成Fe(OH)2、NaAlO2等析出物。进一步在酸浸过程中,Si/S生成的氧化物被溶解,导致少量未溶解石英析出。Fe/Al几乎被去除。揭示了长焰煤生产超低灰分煤过程中Si/S溶解—fe /Al浸出—分步去除机理。该方法制备的超低灰分煤具有较高的应用价值,可为钠离子电池正极和优质活性炭提供原料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
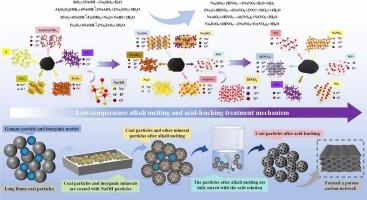
Behavior and mechanism of impurity removal in the process of producing ultra-low ash coal from long-flame coal by low-temperature alkali melting and acid step leaching
Coal, besides diamond and graphite, is the largest source of carbon material in nature. Due to its high chemical reactivity, long-flame coal will be an important precursor of coal-based functional carbon materials. However, coal’s deep desulphurization and ultra-pure preparation have become the main bottlenecks that affect its development. Taking Xinjiang long-flame coal as raw material, the mechanism of removing impurities in long-flame coal to produce ultra-low ash coal by low-temperature alkali melting and acid step leaching was studied. The behavior and mechanism of impurity removal were further explored. The results showed that 0.80 % ash could be extracted during alkali melting, and the yield could reach 73.26 %. After leaching and enrichment with HNO3, the ultra-low ash coal with 0.009 % ash was obtained. The maximum demineralization rate was 99.42 %. The migration mechanism of Si/S/Fe/Al elements was analyzed. Some Si/S elements were removed as oxides, while a small amount of Si/S reacted with NaOH to produce oxides such as Na2SiO3 and Na2S. Fe/Al elements generated Fe(OH)2, NaAlO2, and other precipitates. Further in the acid leaching process, the oxide generated by Si/S was dissolved, resulting in a small amount of undissolved quartz precipitation. Fe/Al was almost removed. The Si/S dissolution-Fe /Al leaching-step removal mechanism in the process of producing ultra-low ash coal from long-flame coal was revealed. The ultra-low ash coal prepared by this method has high application value, which can provide raw materials for the positive electrode of sodium ion batteries and high-quality activated carbon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


