催化不对称分子内烯烃加氢酰化的手性瞬态导向基团
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们开发了一种手性2-氨基吡啶作为Rh(I)的助催化剂来完成高度对映选择性的分子内烯烃加氢酰化反应(高达96:4 er)。手性2-氨基吡啶具有双重作用:醛C-H激活的瞬时导向基团和转化过程中不对称的唯一来源。我们的手性瞬时导向基团方法克服了先前在不对称氢化反应中的局限性,在不对称氢化反应中,需要含有嵌入螯合基团的反应物才能成功。我们展示了我们的方法在对映选择性合成两个生物相关的小分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
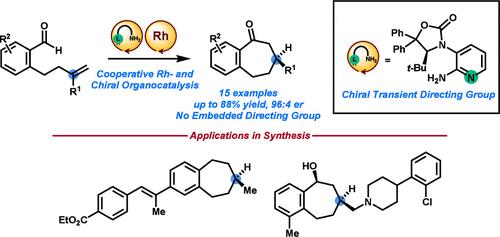
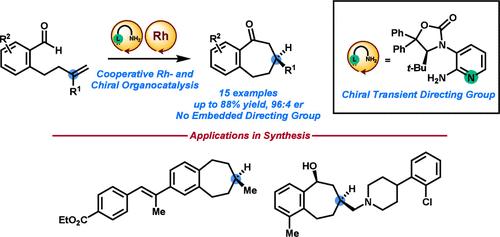
Chiral Transient Directing Groups for Catalytic Asymmetric Intramolecular Alkene Hydroacylation
We developed a chiral 2-aminopyridine as a cocatalyst with Rh(I) to accomplish highly enantioselective intramolecular alkene hydroacylation reactions (up to 96:4 er). The chiral 2-aminopyridine served dual purposes: a transient directing group for aldehyde C–H activation and the sole source of asymmetry in the transformation. Our chiral transient directing group approach overcame prior limitations in asymmetric hydroacylation reactions, where reactants containing embedded chelating groups were required for success. We demonstrated our method in enantioselective syntheses of two biologically relevant small molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


