碳电催化剂超微孔内O2的活化对水污染物的电化学氧化作用
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
电化学氧化是一种有效的水净化技术,但其大规模应用受到能量需求高的限制。本研究通过开发一种超微孔碳(UMC)电催化剂来有效激活分子氧(O2),以协助污染物的电化学氧化,从而解决了这一挑战。O2的参与改变了2,4-二氯苯酚的电化学氧化途径,从聚合到降解,使其在低电位下深度矿化。实验结果和密度泛函理论(DFT)计算将UMC的催化活性归因于sp2-碳共轭和超微孔结构的协同作用。在超微孔的密闭空间中,O2通过从sp2共轭碳壁上捕获电子转化为•OH。超微孔为O2活化提供了一个完全可用的内表面,约束效应增强了O2在sp2共轭碳壁上的吸附和界面电子转移。利用UMC电催化剂,空气增强策略将酚类化合物的电化学氧化能耗降低了50% %以上,并在广泛的pH值和实际水条件下表现出稳定性。这些发现为设计更节能、更少浪费的电化学氧化过程的高效氧活化剂开辟了新的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
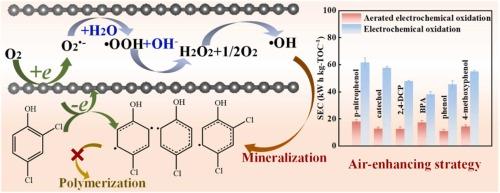
Activation of O2 within confined ultramicropores of carbon electrocatalyst for enhanced electrochemical oxidation of water contaminants
Electrochemical oxidation is effective for water decontamination, but its large-scale application is constrained by high energy demands. This work addresses this challenge by developing an ultramicroporous carbon (UMC) electrocatalyst to effectively activate molecular oxygen (O2) for assisting the electrochemical oxidation of contaminants. Participation of O2 switches the electrochemical oxidation pathway of 2,4-dichlorophenol from polymerization to degradation, allowing its deep mineralization at low potentials. Experimental findings and density functional theory (DFT) calculations attribute the UMC’s catalytic activity to the synergistic sp2-carbon conjugation and ultramicroporous structure. In the confined space of ultramicropores, O2 convert into •OH by capturing electrons from the sp2-conjugated carbon wall. The ultramicropores supplies an inner surface exclusively available for O2 activation, and the confinement effect enhances O2 adsorption to the sp2-conjugated carbon wall and interfacial electron transfer. Taking advantage of the UMC electrocatalyst, the air-enhancing strategy reduces the energy consumption for electrochemical oxidation of phenolic compounds by over 50 %, and demonstrates stability across broad pH and real water conditions. These findings open up new opportunities for designing efficient O2 activators towards more energy-efficient and less wasteful electrochemical oxidation processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


