放射性核素的多路成像
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
核成像为放射性标记化合物的生物分布提供了非侵入性和近定量的洞察,并且具有卓越的灵敏度和几乎无限的穿透深度。这些特性使得核成像在监测药物的药代动力学、生物分布和体内稳定性方面具有很高的价值。此外,放射性探针的多样性允许详细了解细胞动力学,代谢,表观遗传学和其他生物过程。然而,核成像在很大程度上仍然局限于单一示踪剂的研究,或每一种示踪剂的顺序成像。一次只跟踪一个探针或化合物限制了所能获得的洞察力。在此,我们讨论了多种示踪剂同时成像的应用和临床可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
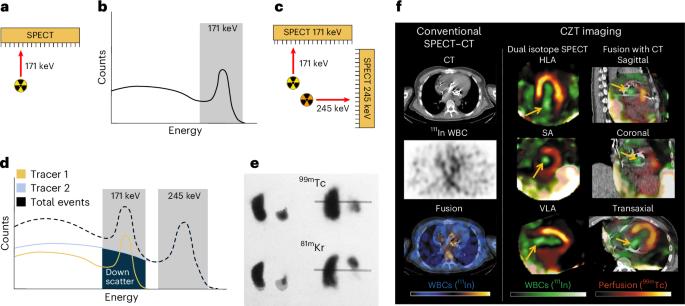

Multiplexed imaging of radionuclides
Nuclear imaging provides non-invasive and near-quantitative insight into the biodistribution of radiolabelled compounds, and it does so with exceptional sensitivity and practically unlimited penetration depth. These properties make nuclear imaging highly valuable for monitoring the pharmacokinetics, biodistribution and in vivo stability of therapeutics. Moreover, the diversity of radioactive probes allows for detailed insight into cell dynamics, metabolism, epigenetics and other biological processes. However, nuclear imaging remains largely limited to single-tracer studies, or to the sequential imaging of each tracer. Tracking only a single probe or compound at a time limits the insight that can be gained. Here we discuss the applications and clinical feasibility of established and upcoming strategies for the simultaneous imaging of multiple radiotracers. This Review discusses the applications and clinical feasibility of established and upcoming strategies for the simultaneous imaging of multiple radiotracers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


