HSP70、HSP90a在温度胁迫下的分子特征、表达响应及生化变化
IF 1.8
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology
Pub Date : 2025-06-17
DOI:10.1016/j.cbpb.2025.111118
引用次数: 0
摘要
本研究探讨了急性温度胁迫下黄鳍鲷(Acanthopagrus latus)热休克蛋白(HSP70、HSP90a和HSP90a1)的分子特征、表达谱及生化变化。克隆并分析了这些热休克蛋白的全长cDNA序列,揭示了分别编码639、730和724个氨基酸的开放阅读框,每个阅读框都含有保守的基序,表明它们在应激反应中的作用。组织特异性表达分析显示,AlHSP70主要在肾脏中表达,而AlHSP90a和AlHSP90a1在健康成年男性的心脏中表达水平最高。在急性温度胁迫下,实时荧光定量PCR (qPCR)结果显示,这些基因在幼鱼肝脏中的表达水平最初升高,随后随着时间的推移逐渐下降并趋于稳定。同时,肝脏中抗氧化酶——总超氧化物歧化酶(T-SOD)和过氧化氢酶(CAT)以及代谢酶乳酸脱氢酶(LDH)的活性也呈现出类似的先升高后下降的模式。这些发现表明,热休克蛋白在温度诱导应激的生理反应中起关键作用,有助于保护拉胡斯免受氧化损伤。本研究为该物种热胁迫驯化的分子机制提供了基本的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
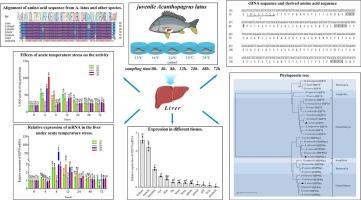
Molecular characterization of HSP70, HSP90a, expression responses and biochemical changes in yellowfin seabream Acanthopagrus latus (Houttuyn 1782) under temperature stress
This study investigates the molecular characteristics and expression profiles of heat shock proteins (HSP70, HSP90a, and HSP90a1) and biochemical changes in yellowfin seabream (Acanthopagrus latus) under acute temperature stress. The full-length cDNA sequences of these HSPs were cloned and analyzed, revealing open reading frames encoding 639, 730, and 724 amino acids, respectively, each containing conserved motifs indicative of their roles in the stress response. Tissue-specific expression analysis showed that AlHSP70 was predominantly expressed in the kidney, while AlHSP90a and AlHSP90a1 exhibited highest expression levels in the heart of healthy adult males. Under acute temperature stress, quantitative real-time PCR (qPCR) demonstrated that hepatic expression levels of these genes in juvenile fish initially increased, followed by a decrease and stabilization over time. Concurrently, activities of antioxidant enzymes—total superoxide dismutase (T-SOD) and catalase (CAT)—and the metabolic enzyme lactate dehydrogenase (LDH) in the liver showed a similar pattern of initial elevation followed by decline. These findings suggest that HSPs play a critical role in the physiological response to temperature-induced stress, contributing to protection against oxidative damage in A. latus. This study provides fundamental insights into the molecular mechanisms underlying thermal stress acclimation in this species.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.60
自引率
4.50%
发文量
77
审稿时长
22 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part B: Biochemical and Molecular Biology (CBPB), focuses on biochemical physiology, primarily bioenergetics/energy metabolism, cell biology, cellular stress responses, enzymology, intermediary metabolism, macromolecular structure and function, gene regulation, evolutionary genetics. Most studies focus on biochemical or molecular analyses that have clear ramifications for physiological processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


