三维n掺杂碳上Fe-Ni双原子协同催化剂高效稳定的电化学CO2还原为CO
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
单原子催化剂(SACs)由于其独特的原子结构和性能特点,在CO₂RR的研究中备受关注。然而,它们固有的缺点,如有限的稳定性和低电荷转移效率,也限制了它们的进一步发展。在本研究中,成功合成了一种新型的Fe-Ni双原子催化剂(Fe2/Ni1-N-C),该催化剂锚定在三维氮掺杂碳框架上,具有显著的电化学活性和将CO2转化为CO的选择性。Fe2/Ni1-N-C催化剂在-0.76 V(相对于RHE)下工作时,其法拉第效率(FECO)高达96%,并在连续工作20 h内保持稳定性能。实验结果表明,在催化剂的原子水平上保持配位环境的稳定性对催化剂的活性、选择性和稳定性起着重要的作用。密度泛函理论(DFT)计算支持相邻Fe-Ni活性位点的成功构建,并伴有NiN4和FeN4的原子弥散。双金属之间的协同作用在促进*COOH中间体的形成和促进*CO的解吸,从而提高CO的选择性和催化活性方面起着关键作用。本研究为设计高效的CO2电还原双原子催化剂提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
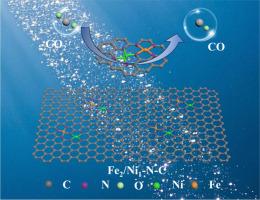
Synergistic Fe-Ni diatomic catalysts on 3D N-doped carbon for high-efficiency and stable electrochemical CO2 reduction to CO
Single-atom catalysts (SACs) have attracted attention in the research of CO₂RR due to their distinct atomic structure and performance features. However, their inherent shortcomings, such as limited stability and low charge transfer efficiency, also limit their further progress. In this study, a novel Fe-Ni diatomic catalyst (Fe2/Ni1-N-C) anchored on a three-dimensional nitrogen-doped carbon framework was successfully synthesized, demonstrating remarkable electrochemical activity and selectivity for converting CO2 to CO. The Fe2/Ni1-N-C catalyst achieved a high Faraday efficiency (FECO) of 96 % when operated at -0.76 V (vs. RHE) and it maintained stable performance over 20 h of continuous operation. The experimental results show that maintaining the stability of the coordination environment at the atomic level of the catalyst plays an important role in its activity, selectivity and stability. Density functional theory (DFT) calculations supported the successful construction of adjacent Fe-Ni active sites, with atomic dispersion of NiN4 and FeN4. The synergistic effect between the bimetals was found to play a critical role in promoting the formation of *COOH intermediates and facilitating the desorption of *CO, thereby enhancing both CO selectivity and catalytic activity. This study provides valuable insights into the design of efficient diatomic catalysts for CO2 electroreduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


