ACAD10和ACAD11促进哺乳动物4-羟基酸脂质分解代谢
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
脂肪酸β-氧化是具有广泛健康意义的核心分解代谢途径。然而,各种脂肪酸,包括4-羟基酸(4-HAs),在被修饰之前,在很大程度上与β-氧化机制不相容。本研究揭示了两种非典型酰基辅酶a脱氢酶ACAD10和ACAD11驱动小鼠4-HA分解代谢。与其他acad不同,ACAD10和ACAD11具有磷酸化4-羟基位置的激酶结构域,这是将4-羟基辅酶a转化为常规2-烯基辅酶a的必要步骤。通过低温电子显微镜和分子模型,我们发现了一个非典型的脱氢酶结合袋,能够容纳这种磷酸化的中间体。我们进一步表明,ACAD10是线粒体的,是分解短链4-HA所必需的,而ACAD11是过氧化物酶体的,可以促进长链4-HA的分解代谢。缺乏ACAD11的小鼠在其血浆中积累4-HAs,雌性小鼠容易体重和脂肪增加,同时脂肪细胞分化和脂肪因子表达减少。总之,我们认为ACAD10和ACAD11是哺乳动物4-HA分解代谢的主要守门人。本文章由计算机程序翻译,如有差异,请以英文原文为准。
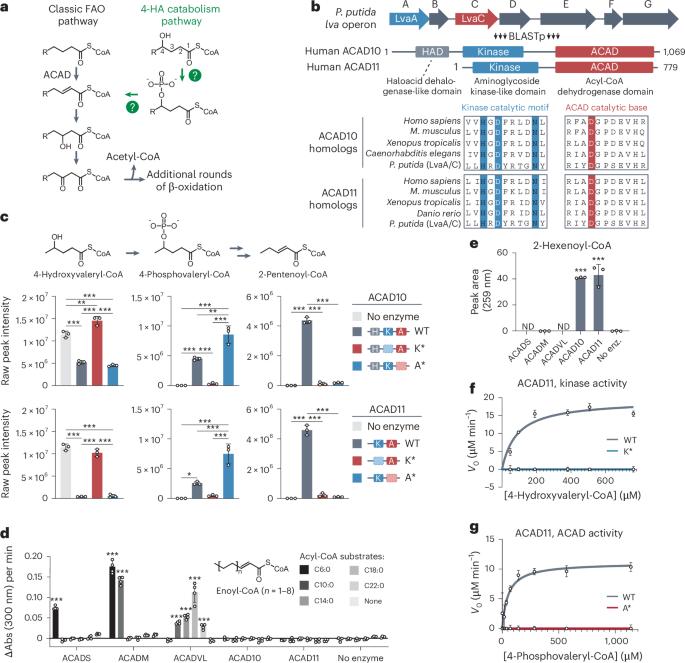
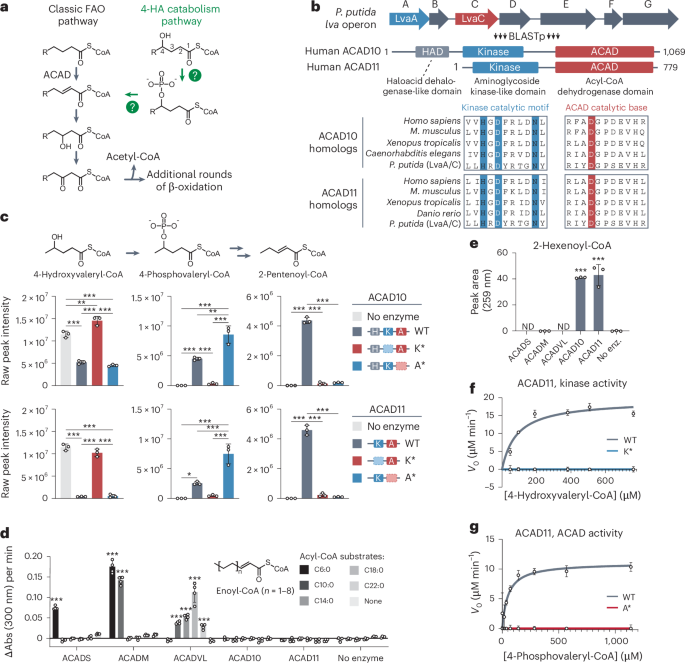
ACAD10 and ACAD11 enable mammalian 4-hydroxy acid lipid catabolism
Fatty acid β-oxidation is a central catabolic pathway with broad health implications. However, various fatty acids, including 4-hydroxy acids (4-HAs), are largely incompatible with β-oxidation machinery before being modified. Here we reveal that two atypical acyl-CoA dehydrogenases, ACAD10 and ACAD11, drive 4-HA catabolism in mice. Unlike other ACADs, ACAD10 and ACAD11 feature kinase domains that phosphorylate the 4-hydroxy position as a requisite step in converting 4-hydroxyacyl-CoAs into conventional 2-enoyl-CoAs. Through cryo-electron microscopy and molecular modeling, we identified an atypical dehydrogenase binding pocket capable of accommodating this phosphorylated intermediate. We further show that ACAD10 is mitochondrial and necessary for catabolizing shorter-chain 4-HAs, whereas ACAD11 is peroxisomal and enables longer-chain 4-HA catabolism. Mice lacking ACAD11 accumulate 4-HAs in their plasma and females are susceptible to body weight and fat gain, concurrent with decreased adipocyte differentiation and adipokine expression. Collectively, we present that ACAD10 and ACAD11 are the primary gatekeepers of mammalian 4-HA catabolism. Rashan, Bartlett and colleagues show that mammalian 4-hydroxy fatty acids are primarily catabolized by ACAD10 and ACAD11 (atypical mitochondrial and peroxisomal acyl-CoA dehydrogenases, respectively) that use phosphorylation in their reaction mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


