含有Cx11NC基序的炭疽菌特有效应物增强植物NDPK2激酶活性以抑制植物免疫
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
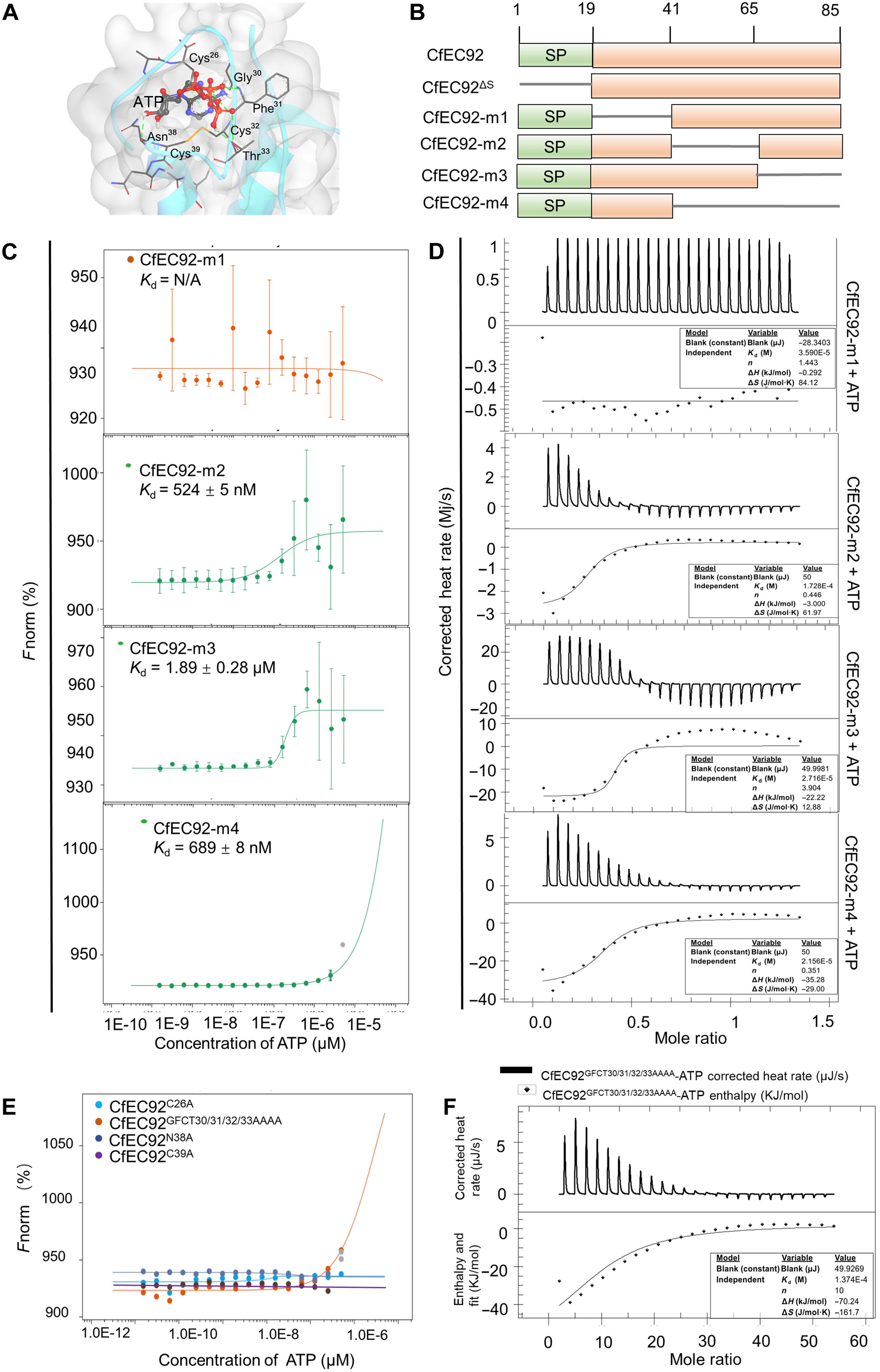
炭疽菌在世界范围内广泛的宿主中引起破坏性疾病。我们发现,来自C. fructicola的效应物CfEC92通过一个未知的ATP结合域特异性地结合ATP,导致蛋白质二级结构的改变。残基Cys26、Asn38和Cys39是ATP与CfEC92结合的关键,这些位点的突变破坏了抑制宿主免疫的能力。CfEC92与苹果负免疫调节因子MdNDPK2相互作用。CfEC92-ATP复合物改变了MdNDPK2的构象,增强了其对ATP的亲和力,并进一步提高了其自磷酸化和激酶活性。激活的MdNDPK2磷酸化MdMPK3抑制宿主免疫。同源性和功能测试表明,Cx11NC基序列在炭疽菌中高度保守,表明CNC效应物是一类广谱毒力因子。本研究揭示了炭疽菌效应物与辅助ATP协同促进靶蛋白磷酸化并抑制宿主免疫的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Colletotrichum-unique effector with the Cx11NC motif enhances plant NDPK2 kinase activity to suppress plant immunity
Colletotrichum fungi cause destructive diseases among a wide range of hosts worldwide. We found that effector CfEC92 from C. fructicola specifically binds ATP through an unidentified ATP-binding domain, leading to changes in the protein secondary structure. The residues Cys26, Asn38, and Cys39 were critical for ATP binding with CfEC92, and mutations at these sites impaired the ability to suppress host immunity. CfEC92 interacted with MdNDPK2, a negative immune regulator in apple. The CfEC92-ATP complex altered the conformation of MdNDPK2, enhancing its affinity for ATP, and further increasing its autophosphorylation and kinase activity. The activated MdNDPK2 phosphorylated MdMPK3 to suppress host immunity. Homology and functional tests showed that the Cx11NC motif was highly conserved among Colletotrichum species, suggesting that CNC effectors represent a class of broad-spectrum virulence factors. Our findings revealed a mechanism by which Colletotrichum effectors cooperate with helper ATP to promote target protein phosphorylation and suppress host immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


