高效去除Pb(II)离子的Mn - Co-BTC@MOF/S-MXene复合材料:机理和DFT研究
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
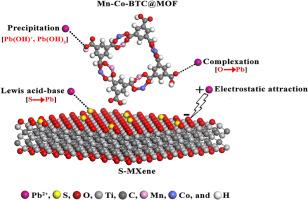
日益严重的水污染需要有效的修复方案。本研究合成了Mn - Co-BTC@MOF/S-MXene复合材料,并通过FTIR, XPS, XRD, SEM和Zeta Potential等多种分析工具对其进行了表征,以证实其制备成功。Zeta电位表明,Mn - Co-BTC@MOF/S-MXene在pH为6时的零电荷点为3.4,表面高度负(- 22.8 mV)。实验结果表明,在最佳条件(pH 6, 298 K)下,30 min内吸附Pb2+的qmax为857.98 mg/g。吸附等温线符合Langmuir模型(R2 = 0.990)和Freundlich模型(R2 = 0.998),表明吸附机制是化学和物理相结合的,并具有准二级动力学模型。复合材料对Pb2+的选择性优于其他竞争离子,顺序为:Pb2+ >;Cd2 +比;Zn2 +比;Cu2 +。有趣的是,DFT计算和Mulliken原子电荷分析表明,硫和氧官能团通过为Pb2+离子提供强结合位点,显著地促进了吸附亲和力。此外,蒙特卡罗和分子动力学模拟进一步支持了这种选择性吸附行为。综上所述,Mn - Co-BTC@MOF/S-MXene是一种高效、选择性和可持续去除水中Pb2+的吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mn–Co-BTC@MOF/S-MXene composite with superior efficiency for Pb(II) ion removal: Mechanistic and DFT study
The increasing severity of water pollution requires effective remediation solutions. In this study, a Mn–Co-BTC@MOF/S-MXene composite was synthesized and characterized by variety of analytical tools including FTIR, XPS, XRD, SEM, and Zeta Potential to confirm its successful fabrication. Zeta potential clarified that Mn–Co-BTC@MOF/S-MXene has a point of zero charge of 3.4 with a highly negative surface (−22.8 mV) at pH 6. Experimental results demonstrated an outstanding Pb2+ adsorption capacity with a qmax of 857.98 mg/g within 30 min under optimal conditions (pH 6, 298 K). The adsorption isotherms followed both Langmuir (R2 = 0.990) and Freundlich (R2 = 0.998) models, indicating the combination of both chemical and physical adsorption mechanisms along with a pseudo-second-order kinetic model. The composite exhibited superior selectivity toward Pb2+ over other competing ions, in the order: Pb2+ > Cd2+ > Zn2+ > Cu2+. Interestingly, DFT calculations and Mulliken atomic charge analysis indicated that sulfur and oxygen functional groups significantly contribute to the adsorption affinity by providing strong binding sites for Pb2+ ions. Moreover, Monte Carlo and molecular dynamics simulations further supported this selective adsorption behavior. In conclusion, these findings revealed that Mn–Co-BTC@MOF/S-MXene is a highly efficient, selective, and sustainable adsorbent for Pb2+ removal from water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


