具有神经分化活性的丁香酚类五萜类化合物
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过对丁香芽的植物化学研究,发现了九种以前未被描述过的基于丁香酚的萜类化合物,命名为丁香醇A-I(1-9)。其中,化合物1是首次报道的丁香酚部分通过两个C-C键与倍半萜单元融合,形成前所未有的三环[9.4.0.04,7]十五烷核心骨架的亚萜类化合物。化合物2-5代表了一类具有前所未有的结构,其特征是丁香酚和倍半萜类之间具有独特的C-C键连接模式。值得注意的是,不寻常的4键导致了罕见的构型稳定的Csp2-Csp3手性轴,同时结合了轴向和点手性。化合物6-9构成了一类未描述的醚桥接丁香酚基巯基萜类化合物。它们的结构通过综合光谱技术、单晶x射线衍射和量子化学计算得以阐明。提出了一种可行的以丁香酚为基础的萜类化合物的生物合成途径。此外,生物学评价显示化合物7显著促进神经分化活性,表明潜在的神经保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
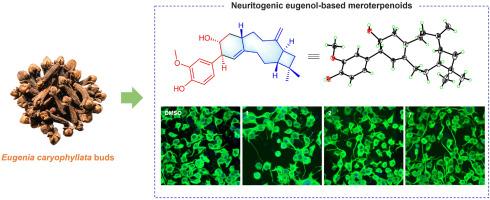
Eugenol-based meroterpenoids with neural differentiation activity from Eugenia caryophyllata
Phytochemical investigation of Eugenia caryophyllata buds led to the discovery of nine previously undescribed eugenol-based meroterpenoids, named eugenianols A–I (1–9). Among them, compound 1 is the first reported meroterpenoid featuring a eugenol moiety fused with a sesquiterpenoid unit via two C–C bonds, forming an unprecedented tricyclo[9.4.0.04,7]pentadecane core skeleton. Compounds 2–5 represent a class of meroterpenoids with unprecedented architectures characterized by distinct C–C bond linkage patterns between the eugenol and sesquiterpenoid moieties. Notably, the unusual linkage of 4 resulted in a rare configurationally stable Csp2–Csp3 chiral axis combining both axial and point chirality. Compounds 6–9 constitute a class of undescribed ether bridged eugenol-based meroterpenoids. Their structures were elucidated through comprehensive spectroscopic techniques, single-crystal X-ray diffraction, and quantum-chemical calculations. A plausible biosynthetic pathway for these eugenol-based meroterpenoids was proposed. Furthermore, biological evaluations revealed that compound 7 significantly promoted neural differentiation-promoting activity, indicating potential neuroprotective properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


