靶向递送IL-10和过氧化氢酶对急性肾损伤的协同治疗作用
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
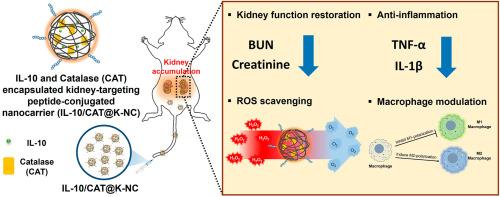
肾脏疾病已成为一个重大的公共卫生问题,影响着全世界约10%的成年人。特别是急性肾损伤(AKI)增加了院内发病率和死亡率。本研究介绍了一种联合抗炎细胞因子白介素-10 (IL-10)和活性氧(ROS)清除酶过氧化氢酶(CAT)治疗炎症性肾损伤的双重治疗策略。开发了一种靶向肽偶联的、基于pluronic的纳米载体(K-NC),以实现IL-10和CAT的共递送和缓释。与无配体共轭的NC相比,K-NC选择性地在受损肾脏中积累,同时减少了肝脏的积累。在体外,CAT可有效降低小管上皮细胞的ROS水平,而IL-10可抑制炎症细胞因子表达,促进M2巨噬细胞极化。在体内,与使用K-NC单独递送IL-10或CAT (IL-10@K-NC或CAT@K-NC)或使用无配体共轭NC (IL-10/CAT@NC)共同递送IL-10和CAT相比,使用K-NC联合递送IL-10和CAT显著增强了AKI的治疗效果,结果显示血清肌酐和BUN水平降低,活性氧减少,促炎细胞因子表达减少,抑制肾M1巨噬细胞极化,促进M2巨噬细胞极化。这项研究不仅提出了一种治疗肾脏炎症的有希望的方法,而且是第一个证明通过抗氧化酶和抗炎细胞因子的组合增强治疗效果的报告。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeted co-delivery of IL-10 and catalase for cooperative therapeutic effect on acute kidney injury
Kidney disease has emerged as a significant public health concern, affecting approximately 10 % of adults worldwide. Especially, acute kidney injury (AKI) increases in-hospital morbidity and mortality. This study introduces a dual-therapeutic strategy combining interleukin-10 (IL-10), an anti-inflammatory cytokine, and catalase (CAT), a reactive oxygen species (ROS)-scavenging enzyme, for the treatment of inflammatory kidney injury. A kidney-targeting peptide-conjugated, Pluronic-based nanocarrier (K-NC) was developed to enable the co-delivery and sustained release of IL-10 and CAT. Compared to no-ligand conjugated NC, K-NC was selectively accumulated in the damaged kidneys while reducing liver accumulation. In vitro, CAT effectively reduced ROS levels in tubular epithelial cells, while IL-10 suppressed inflammatory cytokine expression and promoted M2 macrophage polarization. In vivo, co-delivery of IL-10 and CAT using K-NC resulted in significantly enhanced therapeutic effects on AKI compared to single delivery of IL-10 or CAT using K-NC (IL-10@K-NC or CAT@K-NC), or co-delivery of IL-10 and CAT using no-ligand conjugated NC (IL-10/CAT@NC) by showing reduced levels of serum creatinine and BUN, reduced reactive oxygen species, reduced pro-inflammatory cytokine expression, inhibited M1 macrophage polarization, and promoted M2 macrophage polarization in the kidney. This study not only presents a promising approach for the treatment of kidney inflammation but is also the first report demonstrating enhanced therapeutic effects through a combination of an antioxidant enzyme with an anti-inflammatory cytokine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


