通过组装和共同递送3D打印支架激活骨肿瘤吞噬巨噬细胞
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
目前骨肿瘤术后使用3d打印植入物治疗面临着难以降低复发率的困境,这与植入物无法逆转M2极化以及残留肿瘤细胞导致巨噬细胞丧失肿瘤吞噬能力密切相关。在这项研究中,通过组装一个共递送系统,开发了一种多功能治疗性植入物,该系统在内部多孔结构中富集CSF-1R抑制剂,并基于硼氮配位键将抗sirp抑制剂固定在富含醛的3D打印磷酸钙支架上,从而激活局部肿瘤吞噬巨噬细胞并分阶段促进骨再生。苯基硼酸修饰的介孔二氧化硅纳米颗粒作为一种有效的药物载体,可以富集CSF-1R抑制剂,稳定结合抗体,同时保持其生物活性。通过HBC网络加载,将共递送系统组装到磷酸钙支架上,实现靶向治疗区域的持续释放。这有效阻断了肿瘤细胞与巨噬细胞之间的MCSF/CSF-1R和CD47/SIPR]信号相互作用,从而抑制巨噬细胞的M2极化,同时保持巨噬细胞的吞噬活性,抑制肿瘤复发。之后,组装的结构逐渐解体,暴露出磷酸钙和硅基核心,促进骨组织修复。综上所述,稳定组装的治疗性支架与靶向肿瘤吞噬巨噬细胞原位调控的共递送系统为抑制肿瘤免疫逃逸和术后复发提供了一种新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
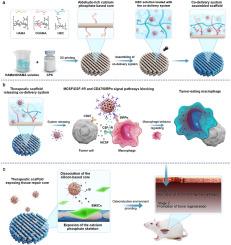
Activation of bone tumor-eating macrophages via assembling and co-delivering 3D printed scaffold
The current post-surgery treatment of bone tumors with 3D-printed implants is facing the dilemma of difficulty in reducing the recurrence rate, which is closely related to the inability of the implants to reverse M2 polarization and the loss of tumor phagocytosis of macrophages caused by residual tumor cells. In this study, a multifunctional therapeutic implant activating local tumor-eating macrophages and promote bone regeneration in stages was developed by assembling a co-delivery system that enriched CSF-1R inhibitors within the internal porous structure and immobilizes anti-SIRPɑ on the surface based on boron-nitrogen coordination bonds onto an aldehyde-rich 3D printed calcium phosphate scaffold using dynamic covalent bonds. The phenylboric acid-modified mesoporous silica nanoparticle served as an efficient drug carrier for the enrichment of CSF-1R inhibitor and stable binding of antibodies while preserving their bioactivity. The co-delivery system was assembled onto the calcium phosphate scaffold through loading via the HBC network, enabling sustained release in the targeted treatment area. This efficiently blocked the MCSF/CSF-1R and CD47/SIPRɑ signaling interactions between tumor cells and macrophages, thereby inhibiting M2 polarization of macrophages while preserving their phagocytic activity and inhibiting tumor recurrence. Afterward, gradual disintegration of the assembled structure exposed the calcium phosphate and silicon-based core, which promoted bone tissue repair. In summary, the stably assembled therapeutic scaffold with a co-delivery system targeting the regulation of tumor-eating macrophages in situ provides a new strategy for the suppression of tumor immune escape and recurrence following surgery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


