芳香单阴离子类卟啉醌脂卟啉及其Cu2+催化降解研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究首次合成并表征了喹啉卟啉(6),这是一种独特的芳香单阴离子大环,在类卟啉核心内含有喹啉单元。以前,含喹啉的卟啉将其喹啉单位定位在外围位置。这种结构特征与Cu2+表现出独特的反应性,导致在室温下μM浓度下30分钟内快速降解并形成荧光产物(7)。这种温和而迅速的反应性归因于喹啉核心的存在。本文章由计算机程序翻译,如有差异,请以英文原文为准。

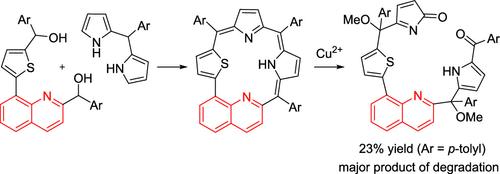
Quinoliporphyrin as an Aromatic Monoanionic Porphyrinoid and Its Degradation Promoted by Cu2+
This study presents the first synthesis and characterization of quinoliporphyrin (6), a unique aromatic monoanionic macrocycle featuring a quinoline unit within the porphyrinoid core. Previously, quinoline-containing porphyrins had their quinoline units positioned at peripheral locations. This structural feature exhibits exclusive reactivity with Cu2+, leading to rapid degradation at μM concentrations within 30 min at room temperature and the formation of a fluorogenic product (7). Such mild and rapid reactivity is attributed to the presence of the quinoline core.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


