Ce7O12在还原气氛中的还原行为和动力学:来自H2和CO比较的见解
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
提出了采用分级焙烧法提高铁稀土分离效果的方法,并进行了中试试验。然而,氟碳铈矿热解产物Ce7O12在还原焙烧阶段的反应鲜有报道。采用热重法研究了Ce7O12在H2和CO作用下的还原反应,并分析了非等温反应动力学。结果表明,由晶格缺陷引起的独特Ce/O使Ce7O12能够与还原性气体发生反应。在H2O和CO2检测下,H2和CO还原过程的失重率分别为2.8 %和2.5 %。H2还原的最佳反应模型为缩核模型(m = 1/2),CO还原的最佳反应模型为化学反应模型(n = 3/2)。在还原过程中,CO还原比H2还原开始温度更低,反应更激烈。还原后,XRD峰向低角度移动,晶格间距增大,Ce氧化程度减小,表明Ce4+向Ce3+转变。还原产物比表面积和总孔容减小,孔径增大。本文章由计算机程序翻译,如有差异,请以英文原文为准。
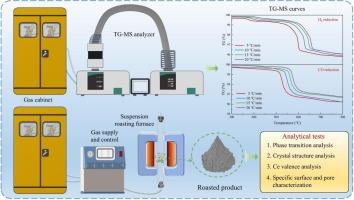
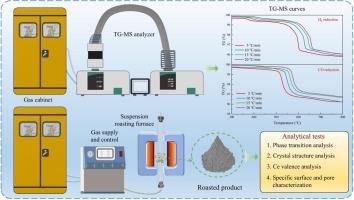
Reduction behavior and kinetics of Ce7O12 in reducing atmospheres: Insights from the comparison of H2 and CO
Enhancing separation of iron and rare earths by staged roasting was proposed, with a pilot-scale experiment conducted. However, the reaction of bastnaesite pyrolysis product (Ce7O12) in reduction roasting stage is rarely reported. This study explored reduction of Ce7O12 under H2 and CO using thermogravimetry, and analyzed the non-isothermal reaction kinetics. The results indicated that the unique Ce/O induced by lattice defects enabled Ce7O12 to react with reducing gases. The weight loss during H2 and CO reduction was 2.8 % and 2.5 %, with the detection of H2O and CO2. The optimal reaction model for H2 reduction was shrinking core model (m = 1/2), while that for CO reduction was chemical reaction model (n = 3/2). During the reduction process, the CO reduction started at a lower temperature than the H2 reduction, and the reaction was more intense. After reduction, the XRD peaks shifted to lower angles, the lattice spacing increased, and the Ce oxidation degree decreased, implying the transition of Ce4+ to Ce3+. The reduction products exhibited a decrease in specific surface area and total pore volume, while the pore diameter increased.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


