菲律宾长叶钩藤中钩藤素A-D、氧吲哚类生物碱及其抗淀粉样蛋白的性质
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从龙花钩藤(Uncaria longiflora, Poir.)的叶子中分离得到8个菊科植物型的氧吲哚类生物碱,包括4个先前未被描述的化合物,uncamarins A-D(1-4)。稳定。(茜草科)产于菲律宾。先前描述的化合物的结构是基于广泛的光谱数据(如NMR, ECD, UV, IR和hresms)来阐明的,而已知的化合物是通过与已发表的文献进行比较来确定的。在100 μg/ml浓度下,采用硫黄素T (ThT)荧光法测定其抗淀粉样蛋白生成活性。所有化合物均表现出抑制活性,其中5(78.44%±1.63)的抑制率最高,其次是4(77.91%±0.22)和2(70.40%±1.93),与阳性对照酚红(82.73±0.84)相当。这些结果得到了分子对接的支持,其中化合物2(−7.5 kcal/mol)、4(−7.1 kcal/mol)和5(−7.2 kcal/mol)与淀粉样蛋白β42的十二聚体结构具有很强的结合亲和力。值得注意的是,化合物4还通过硅对接到乙酰胆碱酯酶的活性位点,乙酰胆碱酯酶是阿尔茨海默病(AD)治疗的关键酶,从而突出了其在阿尔茨海默病(AD)治疗中的多靶向潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
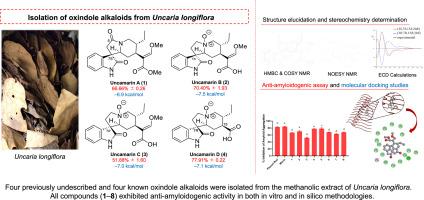
Uncamarins A–D, oxindole alkaloids from Philippine Uncaria longiflora and their anti-amyloidogenic properties
Eight oxindole alkaloids of the Corynanthe-type, including four previously undescribed compounds, uncamarins A–D (1–4), were isolated from the leaves of Uncaria longiflora (Poir.) Merr. (Rubiaceae family) collected in the Philippines. The structures of the previously undescribed compounds were elucidated based on extensive spectroscopic data such as NMR, ECD, UV, IR, and HRESIMS, whereas known compounds were identified by comparison with published literature. Their anti-amyloidogenic activity was evaluated using a thioflavin T (ThT) fluorescence assay at 100 μg/ml. All compounds exhibited inhibitory activity, with 5 (78.44 % ± 1.63) displaying the highest % inhibition, followed by 4 (77.91 % ± 0.22) and 2 (70.40 % ± 1.93), comparable to the positive control, phenol red (82.73 ± 0.84). These results were supported by molecular docking, where compounds 2 (−7.5 kcal/mol), 4 (−7.1 kcal/mol), and 5 (−7.2 kcal/mol) exhibited strong binding affinities to the dodecameric structure of the amyloid-β42 protein. Notably, compound 4 also docked in silico to the active site of acetylcholinesterase, a key enzyme targeted in Alzheimer's disease (AD) therapy, thereby highlighting its potential for multitargeting in AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


