使用fitc -叶酸双特异性适配器桥接CAR免疫细胞鸡尾酒治疗肿瘤的新策略
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
过继性免疫细胞疗法在癌症治疗中显示出希望,但其对实体肿瘤的疗效往往受到免疫抑制肿瘤微环境(TME)的限制。为了克服这些障碍,我们设计了一种创新的免疫细胞鸡尾酒作为组合生物材料平台,利用来自人类多能干细胞(hPSCs)的中性粒细胞和自然杀伤细胞(NK)的互补功能。利用CRISPR/Cas9,我们将一种抗异硫氰酸荧光素(FITC)嵌合抗原受体(CAR)构建物引入到人造血干细胞的AAVS1安全港位点,允许CAR修饰的中性粒细胞和NK细胞分化。这些CAR中性粒细胞表现出强大的抗肿瘤活性,通过双特异性适配器(fitc -叶酸)与肿瘤细胞形成免疫突触,即使在缺氧TMEs中也是如此,而CAR NK细胞则表现出抗原特异性的细胞毒性。由CAR中性粒细胞和CAR NK细胞组成的鸡尾酒生物材料共同产生协同抗肿瘤作用:中性粒细胞增强TME调节,NK细胞提供靶向细胞毒性。这种生物材料为生产CAR中性粒细胞和CAR NK细胞提供了一种可扩展的现成解决方案,可能减少对高剂量外源性细胞因子的需求,并最大限度地减少免疫相关的毒性。我们的研究结果表明,hpsc衍生的CAR中性粒细胞和CAR NK细胞可能形成一种有效的免疫鸡尾酒生物材料,为通过细胞协同作用和TME适应推进实体瘤免疫治疗提供了一种可行的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
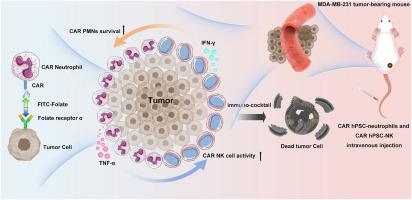
Novel strategy for tumor immunotherapy using FITC-folate bispecific adapter bridged CAR immune cell cocktails
Adoptive immune cell-based therapies have shown promise in cancer treatment, yet their efficacy against solid tumors is often limited by the immunosuppressive tumor microenvironment (TME). To overcome these barriers, we design an innovative immune cell cocktail as a combinatorial biomaterial platform, which harnessing the complementary functions of neutrophils and natural killer (NK) cells derived from human pluripotent stem cells (hPSCs). Using CRISPR/Cas9, we introduce an anti-fluorescein isothiocyanate (FITC) chimeric antigen receptor (CAR) construct into the AAVS1 safe harbor locus of hPSCs, allowing for the differentiation of CAR-modified neutrophils and NK cells. These CAR neutrophils exhibit robust anti-tumor activity, forming immune synapses with tumor cells tagged via a bispecific adapter (FITC-folate), even in hypoxic TMEs, while CAR NK cells demonstrate antigen-specific cytotoxicity. Together, the cocktail biomaterial composed of CAR neutrophils and CAR NK cells creates a synergistic anti-tumor effect: having neutrophils enhance TME modulation, and NK cells provide targeted cytotoxicity. This biomaterial offers a scalable and off-the-shelf solution for producing CAR neutrophils and CAR NK cells, potentially reducing needs for high-dose exogenous cytokines and minimizing immune-related toxicities. Our findings suggest that hPSC-derived CAR neutrophils and CAR NK cells may form an effective immuno-cocktail biomaterial, offering a feasible strategy for advancing solid tumor immunotherapy through cellular synergy and TME adaptation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


