一步法合成去除取代基的下丘脑碱衍生物
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
次氯酸酯是一类天然存在的从真菌中提取的苝丙二烯类色素,由于其独特的光动力学特性而获得了重要的科学兴趣。Hypocrellins具有显著的光激活生物活性,包括有效的抗病毒和抗肿瘤作用,以及良好的药代动力学特性,如快速全身清除。这些有利的性质使hypocrellins成为光动力治疗应用的有希望的候选者。在此,我们报告了一种新的一步策略,有效地合成了两个去除取代基的邻苯二甲酸乙酯衍生物。单晶x射线衍射结构分析表明,hypocrellin 486 (H486)和hypocrellin 426 (H426)保留了苝丙二烯酮的关键结构骨架和七元侧环,消除了天然hypocrellin中的取代基。在光照射下,Hypocrellin衍生物表现出明显的高单线态氧(1O2)量子产率。因此,这些化合物非常适合应用于光动力治疗(PDT)药物靶向癌症或微血管疾病;该合成方法为开发结构优化的过二烯马酮衍生物建立了新的范例,为通过合理的分子设计推进肿瘤和微血管疾病的光动力治疗提供了重要的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
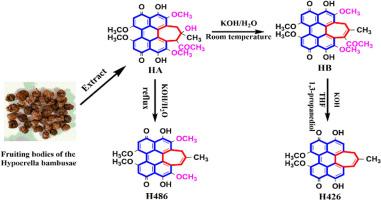
One-step synthesis of hypocrellin derivatives with substituents removed
Hypocrellins, a class of naturally occurring perylenequinone pigments derived from fungi, have garnered significant scientific interest due to their unique photodynamic properties. Hypocrellins exhibit remarkable light-activated biological activities, including potent antiviral and antitumor effects, coupled with favorable pharmacokinetic characteristics such as rapid systemic clearance. These advantageous properties position hypocrellins as promising candidates for photodynamic therapy applications. Herein, we report a novel one-step strategy for the efficient synthesis of two hypocrellin derivatives with removed substituents. Single crystal X-ray diffraction structure analysis reveals hypocrellin 486 (H486) and hypocrellin 426 (H426) retain the key structural skeleton of perylenequinone and the seven-membered side ring, while eliminating substituents in natural hypocrellins. Hypocrellin derivatives demonstrate notably high singlet oxygen (1O2) quantum yields upon photoirradiation. Consequently, these compounds are well-suited for application in photodynamic therapy (PDT) drugs targeting cancer or microvascular diseases; this synthetic methodology establishes a new paradigm for developing structurally optimized perylenequinone derivatives, offering significant prospects for advancing photodynamic therapy in oncology and microvascular disease treatment through rational molecular design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


