1型糖尿病的预防:现在和将来
IF 40
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
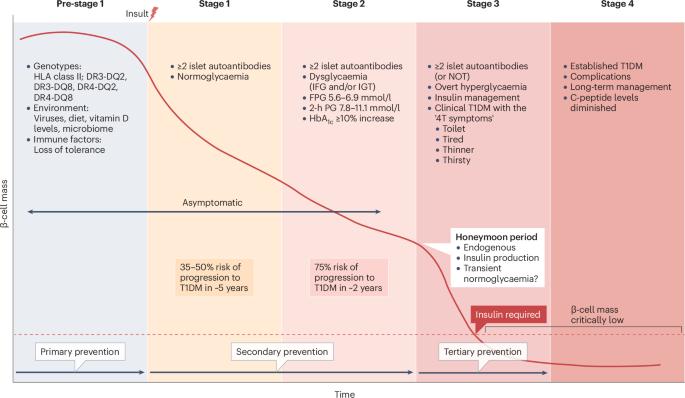
1型糖尿病(T1DM)是一种全球发病率不断上升的慢性疾病。与T1DM并发症相关的死亡率强调了制定预防或延迟T1DM发病的治疗策略的紧迫性。历史上,T1DM仅被描述为一种T细胞介导的疾病。然而,β细胞作为免疫介导损伤的积极参与者的作用现在得到了很好的认识。事实上,异质性、对应激源的脆弱性以及β细胞作为抗原呈递细胞的能力已经改变了有效预防疾病和持续保存β细胞所必需的观点。目前,teplizumab是一种fc受体非结合人源化cd3特异性单克隆抗体,是FDA批准的唯一一种延迟T1DM发病的治疗方法。静脉给药、广泛的免疫抑制和不良反应意味着向常规临床实践的过渡并非没有挑战。然而,teplizumab可能会导致更容易获得的治疗方法的发展。在这篇综述中,我们探讨了目前和潜在的预防T1DM的治疗方法。我们提供替代方法,如靶向晚期糖基化终产物受体(RAGE)。RAGE是一种模式识别受体,涉及广泛的配体,包括晚期糖基化终产物(AGEs;这是一个分子家族,包括长期葡萄糖浓度的标记物HbA1c)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Type 1 diabetes mellitus prevention: present and future
Type 1 diabetes mellitus (T1DM) is a chronic disease with an increasing global incidence. The mortality associated with T1DM complications emphasizes the urgency of developing therapeutic strategies to prevent or delay the onset of T1DM. Historically, T1DM was solely described as a T cell-mediated disease. However, the role of β-cells as active participants in the immune-mediated damage is now well appreciated. Indeed, heterogeneity, vulnerability to stressors and the ability of β-cells to act as antigen-presenting cells has altered the perspective of what is necessary for effective disease prevention and ongoing β-cell preservation. Currently, teplizumab, an Fc-receptor non-binding humanized CD3-specific monoclonal antibody, is the only therapy approved by the FDA for the delay of T1DM onset. The intravenous administration, generalized immunosuppression and adverse effects mean that the transition to routine clinical practice is not without challenges. However, teplizumab could lead to the development of more accessible therapies. In this Review, we explore current and potential therapeutics for T1DM prevention. We offer alternative approaches, such as targeting the receptor for advanced glycation end products (RAGE). RAGE is a pattern recognition receptor that engages a wide range of ligands, including advanced glycation end products (AGEs; a family of molecules that includes the well described marker of long-term glucose concentrations, HbA1c). This Review briefly outlines the pathophysiology of type 1 diabetes mellitus and discusses therapeutic targets that have reached clinical trials, including immunotherapy, and how these treatments might change the future of the disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Endocrinology
医学-内分泌学与代谢
CiteScore
42.00
自引率
0.70%
发文量
158
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Endocrinology aspires to be the foremost platform for reviews and commentaries catering to the scientific communities it serves. The journal aims to publish articles characterized by authority, accessibility, and clarity, enhanced with easily understandable figures, tables, and other visual aids. The goal is to offer an unparalleled service to authors, referees, and readers, striving to maximize the usefulness and impact of each article. Nature Reviews Endocrinology publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians in the fields of endocrinology and metabolism. Its broad scope ensures that the work it publishes reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


