胎盘炎症驱动的T细胞记忆形成通过内源性糖皮质激素促进后代的过敏反应。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
母亲在怀孕期间暴露于环境变化是后代健康和疾病的关键决定因素。先前的流行病学研究报告称,母体炎症与后代出生后过敏发生率增加有关,尽管其潜在机制在很大程度上尚未被探索。在这项研究中,我们采用了脂多糖诱导的母鼠炎症模型,发现母鼠免疫激活(MIA)的后代对屋尘螨过敏原的过敏反应增强。MIA后代显示CD4+ T细胞反应增加,这是通过激活后T细胞存活增加介导的,从而促进中枢和常驻记忆T细胞的形成。在母体炎症期间,TNF-α被确定为驱动后代过敏反应增强的关键细胞因子。TNF-α激活胎盘中性粒细胞,导致胎盘坏死。与胎盘损伤平行,MIA后代在应激反应中表现出糖皮质激素分泌增加。在致敏阶段阻断糖皮质激素通路减轻了MIA后代中增强的T细胞记忆反应,强调了母体炎症可能调节后代免疫反应的机制。我们的研究结果阐明了母体炎症影响后代出生后免疫调节的途径之一。本文章由计算机程序翻译,如有差异,请以英文原文为准。
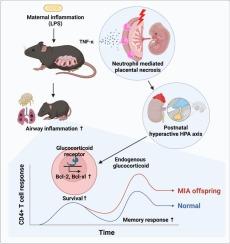
Placental inflammation-driven T cell memory formation promotes allergic responses in offspring via endogenous glucocorticoids
Maternal exposure to environmental change during pregnancy is a critical determinant of offspring health and disease. Previous epidemiological studies have reported that maternal inflammation is linked to an increased incidence of postnatal allergy in offspring, although the underlying mechanisms remain largely unexplored. In this study, we employed a lipopolysaccharide-induced maternal inflammation murine model and found that offspring from dams with maternal immune activation (MIA) exhibited heightened allergic responses to house dust mite allergen. MIA offspring showed an increase in CD4+ T cell responses, which were mediated by increased T cell survival after activation, leading to promoting central and resident memory T cells formation. During maternal inflammation, TNF-α was identified as a crucial cytokine driving the heightened allergic response in offspring. TNF-α activates placental neutrophil, leading to placental necrosis. In parallel with placental damage, MIA offspring demonstrated increased glucocorticoid secretion in response to stress. Blockade of the glucocorticoid pathway during the sensitization phase mitigated the enhanced T cell memory response in MIA offspring, highlighting a mechanism by which maternal inflammation potentially modulates immune responses in offspring. Our findings elucidate one of the pathways by which maternal inflammation can influence postnatal immune regulation in offspring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


