水溶液锌离子电池电解液的改性策略:一种抗溶剂方法。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
锌离子电池(zbs)的发展一直受到与水电解质相关的问题的阻碍,包括析氢、枝晶生长和有限的电化学稳定性。此外,水电解质的分解提出了一个重大的挑战。解决这些障碍的一个可行策略是通过掺入反溶剂对水溶液进行改性,这可以增强ZIBs的电荷存储能力和能量密度。反溶剂的概念在调节内外离子球中起着至关重要的作用,并通过调节溶剂化结构和离子相互作用来增强电解质抑制副反应和减轻锌枝晶形成的能力。这改善了离子插入/脱插入机制,从而提高了循环稳定性。这种改进还扩大了电解质的电化学稳定性窗口,实现了更高的工作电压,并与先进的阴极材料具有更好的兼容性。本文综述了锌离子水溶液电池电解液改性研究的最新进展。主要讨论了azib的优点、面临的重大挑战以及电解质改变的机理。解释了反溶剂的明确选择标准,考虑了它们的性质和溶剂化结构的调制以及它们作为操作条件的函数对性能的影响。最后,从溶剂化鞘和电极/电解质界面的调节角度,阐述了抗溶剂对水溶液电解质影响的机理。此外,本文还讨论了我们在azib的电解质改性领域的贡献,通过反溶剂方法调整电解质结构、输运数、扩散系数、水数及其在溶剂化壳层中的相互作用。这些见解为通过电解质工程实现azib的高性能铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
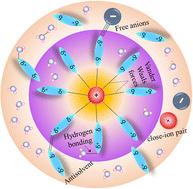
Strategies for electrolyte modification in aqueous zinc-ion batteries: an antisolvent approach
The advancement of zinc-ion batteries (ZIBs) has been impeded by issues associated with the aqueous electrolyte, including hydrogen evolution, dendritic growth, and limited electrochemical stability. Additionally, the decomposition of the aqueous electrolytes presents a significant challenge. A viable strategy to address these impediments involves the modification of aqueous electrolytes through the incorporation of antisolvents, which can enhance the charge storage capability and energy density of ZIBs. The concept of antisolvent plays a crucial role in modulating the inner and outer ionic spheres and enhances the electrolyte's ability to suppress side reactions and mitigate zinc dendrite formation by modulating solvation structures and ionic interactions. This improves the ion insertion/deinsertion mechanism and, subsequently, the cycle stability. This modification also expands the electrolyte's electrochemical stability window, enabling higher operating voltages and better compatibility with advanced cathode materials. This feature review article summarises the recent advancements in electrolyte modification in aqueous Zn-ion batteries (AZIBs). Mainly, the advantages, significant challenges, and mechanism of electrolyte alteration for AZIBs have been discussed. The explicit selection criteria for antisolvents, considering their properties and modulations in the solvation structure and their impact on the performance as a function of operating conditions, are explained. Lastly, the mechanism of the effect of antisolvent on the aqueous electrolyte, considering the regulation of solvation sheath and electrode/electrolyte interface, is described. In addition, our contributions to the field of electrolyte modification for AZIBs with an antisolvent approach to tuning the electrolyte structure, transport number, diffusion coefficient, water numbers and their interaction in the solvation shells are also discussed. These insights pave the way for the realization towards the high performance of AZIBs through electrolyte engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


