转录组学和染色质可及性特征定义了沿脊椎动物轴的颈-胸边界。
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
在脊椎动物胚胎中,沿主体轴在神经管两侧形成一些成对。有些动物产生肌肉骨骼系统的组织,包括脊柱软骨和肋骨以及躯干和四肢的骨骼肌。somite衍生组织的详细解剖结构沿轴线变化,在脊柱中最容易看到独特的特征。在这里,我们研究了这种区域化的遗传控制,它驱动了随后的细胞分化程序,重点是宫颈到胸部(C-T)边界。利用atac测序和rna测序,我们建立了一些体的分子图谱,特别是染色质景观和转录程序,定义了这种解剖转变。差异分析强调候选顺式调控元件(CRE),而硅足迹识别与差异表达基因相关的转录因子(TF)结合位点的覆盖范围。在体内电穿孔黄嘌呤报告基因验证了与HOX关键基因HOXC6和HOXC8相关的cre的活性。HOXC6足迹表明其在调节三个差异表达的SOX转录因子SOX5、SOX6和SOX9中的作用,这些转录因子参与软骨形成。此外,差异分析确定了几个lncrna,包括一个位于HOXC簇中的lncrna。CRISPR-on实验提示HOXC6调控其表达,因此我们将其命名为lncRNA-HOXC6TA,然而其在胸椎区域的功能目前尚不清楚。我们的研究提供了有价值的数据集,并说明了如何挖掘这些数据集,以进一步了解脊椎动物身体轴上C-T转换的调节机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
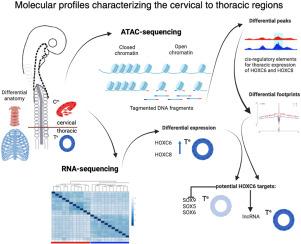
Transcriptomics and chromatin accessibility signatures define the cervical-thoracic boundary along the vertebrate axis
In vertebrate embryos, somite pairs form on either side of the neural tube along the main body axis. Somites generate the tissues of the musculoskeletal system, including cartilage of the vertebral column and ribs and skeletal muscles of the trunk and limbs. The detailed anatomy of somite-derived tissues varies along the axis, with unique features most easily visible in the vertebral column. Here we investigate the genetic control of this regionalization, which drives the subsequent cell differentiation programmes, focusing on the cervical to thoracic (C-T) boundary. Using ATAC-sequencing and RNA-sequencing, we establish molecular profiles of somites, in particular the chromatin landscapes and transcriptional programmes, that define this anatomical transition. Differential analysis highlights candidate cis-regulatory elements (CRE), and in silico footprints identify coverage of transcription factor (TF) binding sites associated with differentially expressed genes. Electroporation of citrine reporters in vivo validates the activity of CREs associated with key HOX genes, HOXC6 and HOXC8. HOXC6 footprints indicate its role in regulating a trio of differentially expressed SOX transcription factors, SOX5, SOX6 and SOX9, which are involved in chondrogenesis. In addition, the differential analysis identifies several lncRNAs, including one that is located within the HOXC cluster. CRISPR-on experiments suggest HOXC6 regulates its expression and therefore we name it lncRNA-HOXC6TA, however, its function in the thoracic region is currently unknown. Our study provides valuable datasets and illustrates how they can be mined to gain further insights into the regulatory mechanisms underlying the C-T transition along the vertebrate body axis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


