pd - l1适配体功能化荧光二氧化硅纳米颗粒治疗三阴性乳腺癌的新系统
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Nanomedicine : nanotechnology, biology, and medicine
Pub Date : 2025-06-13
DOI:10.1016/j.nano.2025.102834
引用次数: 0
摘要
背景三阴性乳腺癌(TNBC)由于缺乏激素受体和HER2表达,缺乏有效的靶向治疗,往往导致预后不良。本研究开发了一种治疗系统,AptPD-L1-FSNPs,将PD-L1适配体与荧光二氧化硅纳米颗粒(FSNPs)结合,用于TNBC的靶向成像和治疗。方法将spd - l1适配体与fsnp结合,形成aptpd - l1 - fsnp。使用pd - l1阳性的TNBC细胞和阴性对照来评估体外结合。利用荧光成像技术评估tnbc小鼠体内肿瘤靶向性和生物分布。通过肿瘤生长抑制、生存和细胞凋亡来衡量治疗效果,并评估主要器官的毒性。结果aptpd - l1 - fsnps对表达pd - l1的TNBC细胞具有高特异性,并延长了肿瘤在体内的停留时间。治疗导致肿瘤生长减少,细胞凋亡增加,以最小的毒性改善生存。结论aptpd - l1 - fsnp具有靶向TNBC成像和治疗潜力,在未来的个性化癌症治疗中具有临床应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
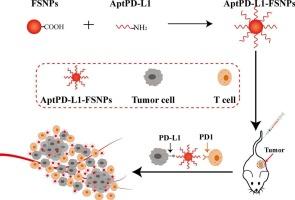
A novel theranostic system of PD-L1-Aptamer-functionalized fluorescent silica nanoparticles for triple-negative breast cancer
Background
Triple-negative breast cancer (TNBC) lacks effective targeted therapies due to absent hormone receptors and HER2 expression, often resulting in poor prognosis. This study developed a theranostic system, AptPD-L1-FSNPs, combining PD-L1 aptamers with fluorescent silica nanoparticles (FSNPs) for targeted imaging and therapy in TNBC.
Methods
PD-L1 aptamers were conjugated to FSNPs, forming AptPD-L1-FSNPs. In vitro binding was evaluated using PD-L1-positive TNBC cells and negative controls. In vivo tumor targeting and biodistribution were assessed via fluorescence imaging in TNBC-bearing mice. Therapeutic efficacy was measured by tumor growth inhibition, survival, and apoptosis, with toxicity assessed in major organs.
Results
AptPD-L1-FSNPs showed high specificity to PD-L1-expressing TNBC cells and prolonged tumor retention in vivo. Treatment led to reduced tumor growth, increased apoptosis, and improved survival with minimal toxicity.
Conclusion
AptPD-L1-FSNPs offer targeted TNBC imaging and therapeutic potential, demonstrating promise for future clinical applications in personalized cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
0.00%
发文量
133
审稿时长
42 days
期刊介绍:
The mission of Nanomedicine: Nanotechnology, Biology, and Medicine (Nanomedicine: NBM) is to promote the emerging interdisciplinary field of nanomedicine.
Nanomedicine: NBM is an international, peer-reviewed journal presenting novel, significant, and interdisciplinary theoretical and experimental results related to nanoscience and nanotechnology in the life and health sciences. Content includes basic, translational, and clinical research addressing diagnosis, treatment, monitoring, prediction, and prevention of diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


