废旧锂离子电池阴极中有价金属的分步回收:深共晶溶剂高效浸出、重结晶和静电相互作用选择性分离
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
随着电动设备和汽车市场的迅速扩大,废旧锂离子电池(LIBs)将大量产生,对环境安全构成严重挑战。锂离子阴极材料中多种有价金属的复杂性和相似性给其提取和分离带来了困难。以氯乙酸(CAA)和盐酸甜菜碱(BeCl)为原料,制备了一种深度共晶溶剂(DES),用于从废阴极材料中提取有价金属。在最佳浸出条件下,Li、Ni、Co和Mn的最大浸出效率分别为99.64 %、99.56 %、98.92 %和99.82 %。此外,乙醇的加入改变了浸出液的极性,导致溶液中[C5H12NO2]+的再结晶。[CoCl4]2−、[MnCl4]2−通过静电相互作用与[C5H12NO2]+形成稳定的化合物,并从浸出液中分离出来。随后,通过添加草酸(OA)和调节ph,逐步选择性沉淀和回收有价金属离子。Ni和Co以NiC2O4·2H2O和CoC2O4·2H2O的形式回收,Mn和Li以Mn(OH)2和Li2CO3的形式回收。CAA和BeCl提供的酸度、还原性和配位能力确保了在相对温和的反应条件下有效地提取有价金属。选择性重结晶和静电相互作用避免了共沉淀的缺点,实现了有价金属的选择性分离。锂阴极中Li、Ni、Co和Mn的总回收率分别为86.1 %、98.78 %、93.15 %和91.97 %。本研究为从废lib中回收有价金属提供了一种绿色的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
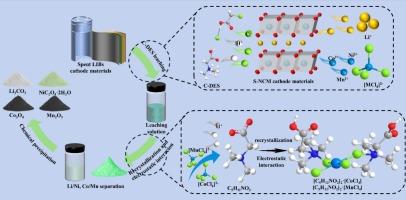
Stepwise recycling valuable metals from spent lithium-ion batteries cathode: high-efficient leaching by deep eutectic solvent and selective separation by recrystallization and electrostatic interaction
With the rapid expansion of electric device and vehicle markets, the huge amount of spent Lithium-ion batteries (LIBs) will be produced and pose a serious challenge to environmental safety. The complexity and similar characteristics for multiple valuable metals presented in LIBs cathode materials result in difficulties in their extraction and separation. In this study, a deep eutectic solvent (DES) was prepared by mixing chloroacetic acid (CAA) and betaine hydrochloride (BeCl), and used to extract valuable metals from spent cathode material. The maximum leaching efficiencies of Li, Ni, Co and Mn meet 99.64 %, 99.56 %, 98.92 % and 99.82 % under optimal conditions. Furthermore, the addition of ethanol alters the polarity of the leaching solution, leading to the recrystallization of [C5H12NO2]+ within the solution. [CoCl4]2−, [MnCl4]2− form stable compounds with [C5H12NO2]+ through electrostatic interaction and were separated from the leaching solution. Subsequently, valuable metal ions were stepwise selectively precipitated and recovered by adding oxalic acid (OA) and adjusting pH. Ni and Co was recovered in NiC2O4·2H2O and CoC2O4·2H2O, Mn and Li was recycled in the form of Mn(OH)2 and Li2CO3. The acidity, reducibility and coordination capabilities provided by CAA and BeCl ensure efficient extraction of valuable metals under relatively mild reaction conditions. The selective recrystallization and electrostatic interaction avoid the shortcomings of co-precipitation, and realize selective separation of valuable metals. The whole recovery efficiencies of Li, Ni, Co and Mn from LIBs cathode achieved around 86.1 %, 98.78 %, 93.15 % and 91.97 %, respectively. This study provides a green method for recovering valuable metals from spent LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


