空气辅助锰/香烟过滤嘴型多孔碳催化剂增强亚硫酸钙氧化的研究:价循环和自由基/非自由基协同途径
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
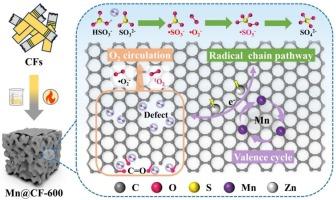
亚硫酸钙(CaSO3)氧化是半干法烟气脱硫过程中钙基脱硫灰(CDA)资源化利用以控制SO2排放的关键步骤。多孔碳基材料因其在亚硫酸盐[S(Ⅳ)]和过硫酸盐(PS)活化的高级氧化反应中的广泛应用而受到广泛关注。本文采用浸渍辅助热解法合成了具有较大SBET (479.16 m2·g−1)和多种活性位点(酮基、Mn种和氧空位)的锰/香烟过滤嘴型多孔碳催化剂(Mn@CF-600),并将其应用于CaSO3的湿式催化氧化。结果表明,在Mn@CF-600空气辅助催化下,CaSO3在3 h内的氧化效率可达91.30%,在338 K时氧化率最高,为0.0566 mmol·L-1·s−1。氧化动力学表明1.0 g·L-1 Mn@CF-600接近扩散控制反应的临界剂量,表观活化能为18.70 kJ·mol−1。空气流速和CaSO3浓度反应级数分别为0.26和- 0.31。此外,固定化CG-Mn@CF-600在5次循环使用后仍保持78.08%的氧化效率。基于淬灭实验、ESR和XPS分析,高稳定性和催化活性可归因于特定活性氧(ROS,包括•SO32-、•O2 -、1O2和•SO5-)、HSO3−和混合价态Mn(II/III/Ⅳ)的协同参与。本研究为碳负载mn基催化剂/CaSO3通过价循环和自由基/非自由基协同途径氧化机理提供了新的见解,从而为CDA的实际处理提供了一种有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insights into the enhanced calcium sulfite oxidation by air assisted with manganese/cigarette filter-based porous carbon catalysts: Valence cycle and radical/non-radical synergistic pathways
Calcium sulfite (CaSO3) oxidation represents a crucial step in the resource utilization of calcium-based desulfurization ash (CDA) from semidry flue gas desulfurization processes for SO2 emission control. Porous carbon-based materials for heterogeneous catalytic oxidation have received attention due to their frequent applications in advanced oxidation reactions involving sulfites [S(Ⅳ)] and persulfates (PS) activation. Herein, a manganese/cigarette filter-based porous carbon catalysts (Mn@CF-600) with a large SBET (479.16 m2·g−1) and various active sites (ketone group, Mn species, and oxygen vacancies) was synthesized by the impregnation-assisted pyrolysis method and applied to wet catalytic oxidation of CaSO3. The results showed that an oxidation efficiency of CaSO3 up to 91.30 % within 3 h were achieved under the air-assisted catalysis of Mn@CF-600, and the highest oxidation rate was 0.0566 mmol·L-1·s−1 at 338 K. Oxidation kinetics indicated that 1.0 g·L-1 Mn@CF-600 approached the critical dosage for diffusion-controlled reactions, with an apparent activation energy of 18.70 kJ·mol−1. The air flow rate and CaSO3 concentration reaction orders were 0.26 and −0.31, respectively. Moreover, the immobilized CG-Mn@CF-600 maintained an oxidation efficiency of 78.08 % after five cycles of use. Based on quenching experiments, ESR, and XPS analysis, the high stability and catalytic activity could be attributed to the synergistic involvement of specific reactive oxygen species (ROS, including •SO32-, •O2–, 1O2, and •SO5-), HSO3−, and mixed valence Mn(II/III/Ⅳ). This work provides new insights into the carbon-supported Mn-based catalyst/CaSO3 oxidation mechanism by the valency cycle and radical/non-radical synergistic pathway, and hence develops an efficient approach for practical CDA treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


