免疫系统动力学对铜绿假单胞菌生物膜的反应。
IF 9.2
1区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
铜绿假单胞菌生物膜通过抵抗免疫攻击和抗生素导致慢性感染。这篇综述探讨了包括中性粒细胞、巨噬细胞和树突状细胞在内的先天免疫如何对生物膜做出反应,以及包括T细胞、B细胞和免疫球蛋白在内的适应性机制如何促进感染的持续存在。此外,它还强调了免疫逃避策略,并讨论了新兴疗法,如免疫疗法、单克隆抗体和疫苗,为增强生物膜清除和改善治疗结果提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
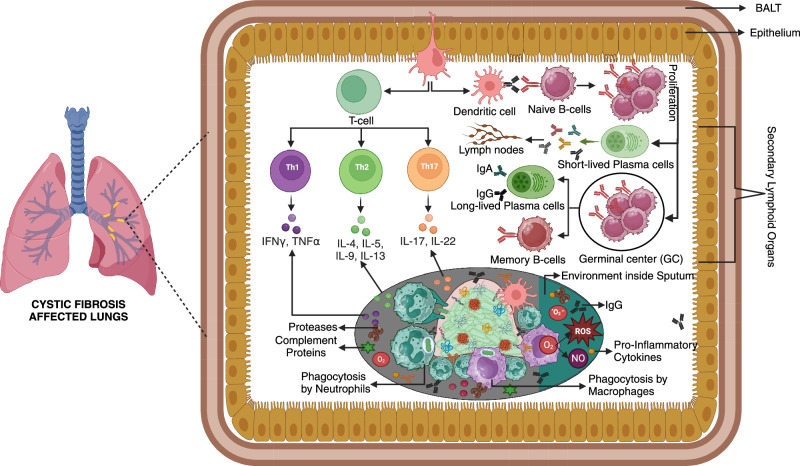
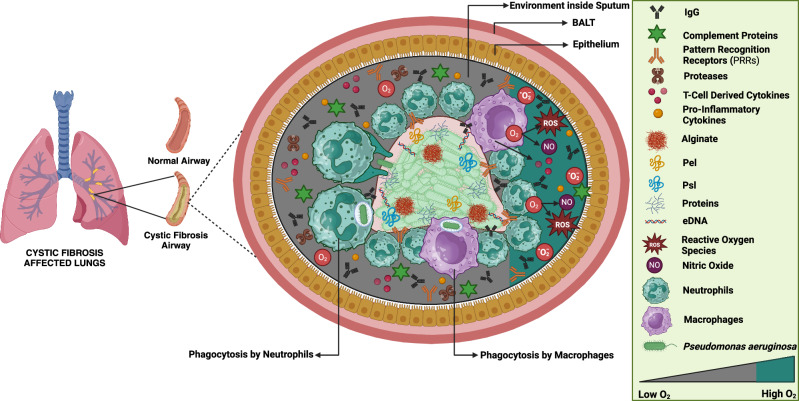
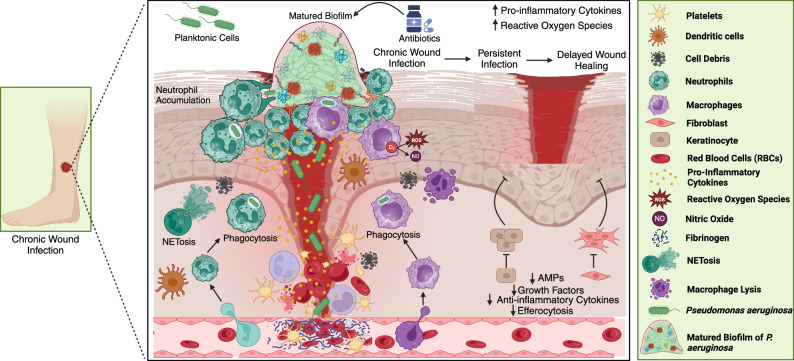
Immune system dynamics in response to Pseudomonas aeruginosa biofilms.
Pseudomonas aeruginosa biofilms contribute to chronic infections by resisting immune attacks and antibiotics. This review explores how innate immunity, including neutrophils, macrophages, and dendritic cells, responds to biofilms and how adaptive mechanisms involving T cells, B cells, and immunoglobulins contribute to infection persistence. Additionally, it highlights immune evasion strategies and discusses emerging therapies such as immunotherapy, monoclonal antibodies, and vaccines, offering insights into enhancing biofilm clearance and improving treatment outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

npj Biofilms and Microbiomes
Immunology and Microbiology-Microbiology
CiteScore
12.10
自引率
3.30%
发文量
91
审稿时长
9 weeks
期刊介绍:
npj Biofilms and Microbiomes is a comprehensive platform that promotes research on biofilms and microbiomes across various scientific disciplines. The journal facilitates cross-disciplinary discussions to enhance our understanding of the biology, ecology, and communal functions of biofilms, populations, and communities. It also focuses on applications in the medical, environmental, and engineering domains. The scope of the journal encompasses all aspects of the field, ranging from cell-cell communication and single cell interactions to the microbiomes of humans, animals, plants, and natural and built environments. The journal also welcomes research on the virome, phageome, mycome, and fungome. It publishes both applied science and theoretical work. As an open access and interdisciplinary journal, its primary goal is to publish significant scientific advancements in microbial biofilms and microbiomes. The journal enables discussions that span multiple disciplines and contributes to our understanding of the social behavior of microbial biofilm populations and communities, and their impact on life, human health, and the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


