木质素合成具有生物活性吲哚的模块化合成路线。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二醇辅助分馏已成为一种重要的“木质素优先”处理方法,以高选择性提供芳香c2 -缩醛。这一贡献描述了一种意想不到的直接合成途径的发展,从这种芳香平台化学物质到生物活性吲哚,扩大了这种独特的生物炼制方法的范围。该新方法利用苯酚烷基化和轻度卤化反应将c2 -缩醛功能化,从而催化C-N与苯胺和苄胺偶联并生成邻氨基缩醛中间体。这些衍生物适合在甲醇/水和PTSA作为催化剂的混合物中通过缩醛脱保护进行原位席夫碱形成/分子内环化,从而形成一个新的木质素基吲哚库。利用人类Hep G2细胞对其抗癌活性进行的生物活性评估显示出有希望的早期结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
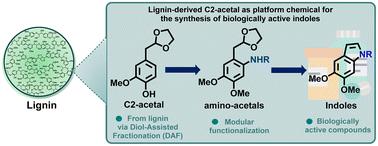
Modular synthetic routes to biologically active indoles from lignin†
Diol-assisted fractionation has emerged as an important ‘lignin-first’ processing method that delivers aromatic C2-acetals with high selectivity. This contribution describes the development of an unexpectedly straightforward synthetic route to biologically active indoles from this aromatic platform chemical, boosting the scope of this unique biorefinery approach. The novel method utilizes the functionalization of C2-acetal via phenol alkylation and mild halogenation reactions, enabling catalytic C–N coupling with anilines and benzylamines and forging ortho-aminoacetal intermediates. Such derivatives are suitable for in situ Schiff base formation/intramolecular cyclization by acetal deprotection in a mixture of MeOH/H2O and PTSA as the catalyst, resulting in a novel library of lignin-based indoles. Evaluation of the biological activity in terms of anticancer activity using human Hep G2 cells shows promising early results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


