利西替尼靶向胰岛素样生长因子1信号通路调节成纤维细胞可塑性,减轻肺纤维化
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
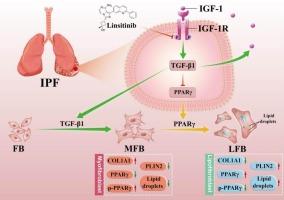
特发性肺纤维化(IPF)是一种致命的、不可逆的肺部疾病,治疗方案有限。致病因素包括成纤维细胞向肌成纤维细胞转化、过多的胶原沉积和炎症浸润。循环胰岛素样生长因子-1 (IGF-1)水平升高和IGF-1受体(IGF-1R)激活失调与多种病理状况有关,如癌症、慢性炎症和纤维化疾病。Linsitinib是一种小分子酪氨酸激酶抑制剂,选择性靶向IGF-1R激活。然而,其在IPF中的治疗潜力和药理机制仍未被探索。在本研究中,我们旨在评价林西替尼治疗IPF的疗效和分子基础。高通量测序显示,小鼠IPF模型肺组织中IGF-1表达上调。在体内,口服利西替尼可减轻博莱霉素诱导的肺纤维化的纤维化和炎症,抑制IGF-1R磷酸化。在体外,林西替尼抑制转化生长因子β1诱导的原代肺成纤维细胞向肌成纤维细胞转变(以α -平滑肌肌动蛋白和纤维连接蛋白减少为标志)和胶原生物合成(COL1A1, COL3A1)。转录组学分析和基于慢病毒的功能分析表明,林西替尼通过阻断IGF-1R磷酸化上调过氧化物酶体增殖物激活受体γ (PPARγ)的表达,从而促进脂肪的转分化。体外实验证实,林西替尼通过IGF-1/IGF-1R/PPARγ轴减轻了纤维化和胶原积累。这些发现表明,Linsitinib通过靶向igf - 1r驱动的信号通路来发挥其抗纤维化作用,使其成为IPF的潜在治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting insulin-like growth factor 1 signaling with Linsitinib to modulate fibroblast plasticity and attenuate pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a fatal, irreversible lung disorder with limited treatment options. Pathogenic drivers include fibroblast-to-myofibroblast transition, excessive collagen deposition, and inflammatory infiltration. Elevated circulating insulin-like growth factor-1 (IGF-1) levels and dysregulated activation of the IGF-1 receptor (IGF-1R) are implicated in multiple pathological conditions such as cancer, chronic inflammation, and fibrotic diseases. Linsitinib, a small-molecule tyrosine kinase inhibitor, selectively targets IGF-1R activation. However, its therapeutic potential and pharmacological mechanisms in IPF remain unexplored. In this study, we aimed to evaluate the efficacy and molecular basis of Linsitinib for IPF treatment. High-throughput sequencing revealed IGF-1 upregulation in the lung tissues of a murine IPF model. In vivo, oral Linsitinib attenuated fibrosis and inflammation in bleomycin-induced pulmonary fibrosis, inhibiting IGF-1R phosphorylation. In vitro, Linsitinib suppressed transforming growth factor β1-induced fibroblast-to-myofibroblast transition (marked by reduced alpha smooth muscle actin and fibronectin) and collagen biosynthesis (COL1A1, COL3A1) in primary lung fibroblasts. Transcriptomic profiling and lentiviral-based functional assays demonstrated that Linsitinib upregulated peroxisome proliferator-activated receptor gamma (PPARγ) expression by blocking IGF-1R phosphorylation, thereby promoting adipogenic transdifferentiation. Ex vivo, human IPF lung explants confirmed Linsitinib mitigated fibrosis and collagen accumulation via the IGF-1/IGF-1R/PPARγ axis. These findings suggest that Linsitinib exerts its effects against fibrosis by targeting IGF-1R-driven signaling pathways, making it a potential therapeutic agent for IPF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


