揭示矮芽孢杆菌促进植物生长的生化途径的遗传基础,并首次从基因组角度了解假菌芽孢杆菌作为生物刺激素的作用
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
芽孢杆菌由于其对各种环境的适应性和广泛的生物合成能力而成为最有前途的植物生长促进菌(PGPB)之一。尽管有关于芽孢杆菌pgp性状(PGPTs)的可用数据,但其有益作用的遗传基础仍未得到很大程度的探索。本研究对从玉米根际分离的3株矮矮型双歧杆菌和1株假菌双歧杆菌进行了比较基因组分析,以阐明其pgp性状的分子机制。所有菌株都表现出多种pgp性状,包括磷酸盐溶解、植物激素和铁载体的产生、无氮培养基的生长、胁迫耐受性和生物膜的形成。系统基因组分析显示,植物相关菌株具有较高的遗传相似性,强调小生境特异性进化。基因组分析揭示了菌株和物种特异性适应,特别是与营养获取和非生物应激反应机制有关。杆状芽孢杆菌菌株编码替代sigma因子(SigB, SigM, SigW),增强耐盐性,而假芽孢杆菌缺乏该系统,依赖于传统的渗透保护策略。菌株利用不同的色氨酸依赖(IAN, IAM或IPyA)途径进行生长素的生物合成,并且在磷酸盐溶解能力上存在差异,这可归因于影响酸代谢(gltA, acnA, acnB, citM和citS)和磷酸酶(phoA)活性的基因的上游和错义变异。通过杆菌杆菌素-铁载体对铁的吸收是扁杆菌所独有的。假芽孢杆菌菌株无法获得铁与细菌合成基因簇内的结构变异(缺乏bsaA基因)有关。这项工作为芽孢杆菌PGP特性的分子基础提供了新的见解,并为芽孢杆菌类生物接种剂的可持续农业发展提供了支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
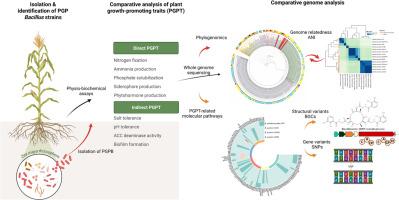
Unveiling the genetic basis of biochemical pathways of plant growth promotion in Bacillus pumilus and the first genomic insights into B. pseudomycoides as a biostimulant
Bacillus species are among the most promising plant growth-promoting bacteria (PGPB) due to their adaptability to various environmental niches and extensive biosynthetic capabilities. Despite the available data on the PGP-traits (PGPTs) of Bacillus, the genetic basis underlying their beneficial effects remains largely unexplored. In this study, a comparative genomic analysis of three B. pumilus and one B. pseudomycoides strains, isolated from the maize rhizosphere, is presented to elucidate the molecular mechanisms behind their PGP-traits. All strains exhibited multiple PGP-traits, including phosphate solubilization, phytohormone and siderophore production, growth in nitrogen-free medium, stress tolerance, and biofilm formation. Phylogenomic analysis revealed that plant-associated strains have higher genetic similarity, emphasizing niche-specific evolution. Genome analyses revealed strain- and species-specific adaptations, particularly in relation to nutrient acquisition and abiotic stress response mechanisms. B. pumilus strains encoded alternative sigma factors (SigB, SigM, SigW) enabling enhanced salt tolerance, whereas B. pseudomycoides lacked this system and relied on conventional osmoprotective strategies. The strains utilized different tryptophan-dependent (IAN, IAM or IPyA) pathways for auxin biosynthesis and differed in phosphate solubilization ability, which can be attributed to upstream and missense variants in genes affecting acid metabolism (gltA, acnA, acnB, citM, and citS) and phosphatase (phoA) activity. Iron uptake via bacillibactin-siderophores was exclusive to B. pumilus. The inability of the B. pseudomycoides strain to acquire iron was associated with structural variants (absence of bsaA gene) within the bacillibactin biosynthetic gene cluster. This work provides new insights into the molecular basis of PGP traits in Bacillus and supports the development of Bacillus-based bioinoculants for sustainable agriculture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


